Myotonic dystrophy type 1 (DM1) is a multisystem disease in which cardiac involvement is common. The aim of this study was to identify early changes in left atrial (LA) mechanics and left ventricular (LV) systolic function in patients with myotonic dystrophy type 1 using three-dimensional (3D) speckle tracking echocardiography (3D-STE).
MethodsThis observational study included 25 patients with DM1 and 25 healthy volunteers. We assessed LA and LV global strain parameters using 3D-STE.
ResultsPatients with DM1 showed significantly lower longitudinal LA strain (22.85%±5.06 vs. 26.82%±5.15; p=0.008 in univariate analysis and p=0.026 in multivariate analysis) and global LV longitudinal strain (-13.55%±1.82 vs. -16.11%±1.33; p<0.001 in univariate analysis and p<0.001 in multivariate analysis), which was not observed with LA area tracking (p=0.412) or LV global circumferential strain (p=0.879), global radial strain (p=0.058), area tracking (p=0.092) or twist (p=0.992).
ConclusionLA and LV global longitudinal strain is significantly decreased in patients with DM1, which may be an early marker of subclinical dysfunction in these patients.
A distrofia miotónica do tipo 1 é uma doença multissistémica com o envolvimento cardíaco a ser comum. O objetivo deste estudo foi identificar alterações precoces no strain da aurícula esquerda e função sistólica do ventrículo esquerdo em doentes com distrofia miotónica tipo 1, com o uso da tecnologia 3D speckle tracking.
MétodosEstudo observacional, que incluiu 25 doentes com distrofia miotónica do tipo 1 e 25 voluntários saudáveis. Foram avaliados os parâmetros de deformação global da aurícula esquerda e do ventrículo com o uso de 3D speckle tracking.
ResultadosOs doentes com distrofia miotónica do tipo 1 apresentaram strain longitudinal da aurícula esquerda (22,85%±5,06 versus 26,82%±5,15; p=0,008 na análise univariada e p=0,026 na análise multivariada) e strain global longitudinal do ventrículo esquerdo (-13,55%±1,82 versus -16,11%±1,33, p<0,001 na análise univariada e p<0,001 na análise multivariada) significativamente menores, o que não se verificou com a area tracking da aurícula esquerda (p=0,412) ou com o strain global circunferencial (p=0,879), strain global radial (p=0,058), area tracking (p=0,092) e twist (p=0,992) do ventrículo esquerdo.
ConclusãoO strain longitudinal global do ventrículo esquerdo e da aurícula esquerda encontra-se significativamente diminuído em doentes com distrofia miotónica do tipo 1, pode ser um marcador precoce de disfunção subclínica nesaes doentes.
myotonic dystrophy type 1
left atrial
left ventricular
three-dimensional
speckle tracking echocardiography
two-dimensional
longitudinal strain
area tracking
global longitudinal strain
global circumferential strain
global radial strain
intraclass correlation coefficient
Myotonic dystrophy type 1 (DM1) is a multisystem neuromuscular disease with autosomal dominant transmission that affects smooth and skeletal muscle.1 About 30% of mortality in adults with DM1 is due to cardiac involvement.2 Arrhythmias, conduction system disturbances and myocardial fibrosis are the most frequent changes.3 Detection of early markers may facilitate the identification of patients at risk of developing cardiac complications.
Left atrial (LA) function is a complex process that includes receiving pulmonary venous return during left ventricular (LV) systole, transfer of blood passively into the left ventricle in early diastole, and active contraction in late diastole.4,5
In a comparison of volumetric real-time three-dimensional (3D) echocardiography and 3D speckle tracking echocardiography (3D-STE), the two methods were found to provide comparable and reproducible measurements, and to be interchangeable for quantification of LA dimensions and functional properties.6
Echocardiographic myocardial tracking systems using 3D-STE have provided new insights into ventricular function and atrial mechanics, with many potential clinical applications.7,8 3D formats more closely represent reality than two-dimensional (2D) formats, not only in the assessment of LV function but also in the diagnosis of valvular or myocardial disease. Determination of LV strain by 2D speckle tracking echocardiography (2D-STE) enables identification of changes in LV systolic function in patients with DM1.9,10 However, the applicability of 3D-STE in these patients is not fully established and the available data are scarce.
The aim of this study was to identify early changes in LA mechanics and LV systolic function in patients with DM1 using 3D-STE.
MethodsStudy populationThis was a single-center observational study. After written informed consent was obtained, all 25 patients with DM1 followed in our center and 25 healthy volunteers (control group) were consecutively selected. All DM1 patients had genetic confirmation of the diagnosis and had no known cardiovascular disease, but had signs of neuromuscular involvement (myopathic facies, myotonia or gait abnormalities).
Echocardiographic protocolThe equipment used was an Artida™ scanner (Toshiba Medical Systems). The echocardiographic study began with a 2D echocardiogram following the standard protocol used in our department, measurements being made in accordance with the current guidelines of the American Society of Echocardiography,11 using a PST-30SBT 1-5 MHz phased-array transducer.
For 3D echocardiography a PST-25SX 1-4 MHz phased-array matrix transducer was used. Images were acquired in apical 4-chamber and 2-chamber views for both LA and LV assessment. The data set is displayed in five planes: apical 4-chamber, apical 2-chamber, and three short-axis planes: apical region, middle segments and basal portion. The software is semiautomatic: it divides the left atrium and ventricle into 16 segments automatically, and after the markers have been selected, the system performs wall motion tracking analysis, enabling analysis of the study variables. The reference values for this equipment for 3D LV strain are: global longitudinal strain (GLS), -15.9%±2.4; global circumferential strain (GCS), -30.6%±2.6; global radial strain (GRS), 35.6%±10.3; area tracking (AT), -42%±2.4.12
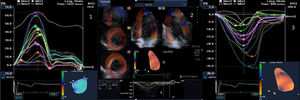
Variables analyzedAll patients were assessed for the presence of neuromuscular manifestations (particularly myotonia, muscular weakness and modified Rankin Scale [mRS] score) and of sleep, respiratory and gastrointestinal disorders, as established in the various consultations in which the patients were followed. Regarding electrocardiographic variables, rhythm, PR interval duration and QRS duration were recorded. On 2D echocardiography, LA size and volume, LV size and diastolic function and tricuspid annular plane systolic excursion (TAPSE) were determined, while LA emptying fraction and end-systolic and end-diastolic volumes and LV ejection fraction (LVEF), end-systolic and end-diastolic volumes and mass were determined by 3D echocardiography. LA deformation was determined by measuring longitudinal strain (LS) and AT, while LV deformation was determined by measuring GLS, GCS, GRS, AT and twist by 3D-STE (Figure 1).
(1) LA LS (16 segments) obtained by means of 3D wall-motion tracking technology; during systole, the LA is stretched and LS increases, reaching a positive peak at the end of systole, corresponding to the end of atrial filling; during diastole, LS decreases to a plateau; (2) the 3D echocardiographic dataset is displayed in apical 4-chamber (A) and 2-chamber (B) views and three short-axis views in the basal (C3), mid-ventricular (C5), and superior (C7) regions, respectively. 3D LV cast (D) and GLS (E) are also presented; (3) LV GLS (16 segments) obtained by 3D wall-motion tracking technology. 3D: three-dimensional; GLS: global longitudinal strain; LA: left atrial; LS: longitudinal strain; LV: left ventricular.
Data sets from the wall motion tracking results were analyzed at different times by two independent observers. The same images were also analyzed on different days by one of these observers. Blinding was ensured by analyzing the studies on different days by the same observer in a randomized order and by blinding the results between the two independent observers.
Statistical analysisStatistical analysis was carried out using SPSS version 22.0. Variables were analyzed for normality of distribution using the Kolmogorov-Smirnov test. Quantitative data are expressed as mean ± SD and qualitative data as percentages. Comparisons were assessed using paired-samples t tests for quantitative data. Cox regression analysis was performed for multivariate adjustment. Correlations were assessed by simple linear regression analysis. Interobserver and intraobserver reproducibility were assessed by means of the intraclass correlation coefficient (ICC). Differences were considered statistically significant with a p-value <0.05.
ResultsClinical and electrocardiographic dataMean age was 36.9±16.0 years in DM1 patients (40.3±12.4 years in controls). All patients had myotonia and 11 had muscle weakness, with a mean mRS of 1.25. Ten patients had PR interval >200 ms and seven had QRS > 120 ms. Clinical and electrocardiographic data are displayed in Table 1.
Clinical and electrocardiographic data.
| DM1 patients | Controls | p | |
|---|---|---|---|
| Age (years) | 36.9±16.0 | 40.3±12.4 | 0.241 |
| Female gender (%) | 56 | 52 | 0.759 |
| Sinus rhythm (n) | 25 | 25 | NA |
| PR interval (ms) | 193±32 | 180±24 | 0.114 |
| QRS (ms) | 116±29 | 106±15 | 0.346 |
| Myotonia (n) | 25 | N | NA |
| Muscle weakness (n) | 11 | N | NA |
| Mean mRS | 1.25 | N | NA |
| Sleep disorders (n) | 2 | N | NA |
| Respiratory disorders (n) | 1 | N | NA |
| Gastrointestinal disorders (n) | 1 | N | NA |
Values are mean ± standard deviation.
DM1: myotonic dystrophy type 1; mRS: modified Rankin Scale score; N: normal; NA: not applicable.
Two-dimensional echocardiography did not identify differences between the groups in left cardiac chamber size or in parameters of diastolic function (Table 2).
Two- and three-dimensional echocardiographic data.
| DM1 patients | Controls | p | |
|---|---|---|---|
| 2D echocardiography | |||
| LA diameter (mm) | 33.10±2.98 | 34.64±3.48 | 0.110 |
| LAV/BSA (ml/m2) | 23.68±5.22 | 26.77±6.09 | 0.070 |
| LVEDD (mm) | 45.12±4.94 | 47.53±3.99 | 0.064 |
| IVS (mm) | 8.96±1.34 | 9.68±1.52 | 0.082 |
| PW (mm) | 8.28±1.31 | 8.96±1.43 | 0.086 |
| E/A | 1.70±0.64 | 1.48±0.44 | 0.155 |
| dT (ms) | 197.36±47.89 | 172.84±41.90 | 0.058 |
| E/E′ | 6.04±2.32 | 5.40±1.52 | 0.306 |
| TAPSE (mm) | 21.42±3.42 | 21.83±3.65 | 0.725 |
| 3D echocardiography | |||
| LAEF (%) | 42.13±8.53 | 46.37±11.21 | 0.139 |
| LAESV/BSA (ml/m2) | 13.12±4.35 | 13.12±3.50 | 0.569 |
| LAEDV/BSA (ml/m2) | 22.70±4.75 | 23.60±6.19 | 0.677 |
| LA LS (%) | 22.85±5.06 | 26.82±5.15 | 0.008 |
| LA AT (%) | 21.77±17.49 | 25.41±12.94 | 0.412 |
| LVEF (%) | 62.88±3.27 | 65.64±6.65 | 0.80 |
| LV mass/BSA (g/m2) | 91.83±21.51 | 85.80±15.73 | 0.267 |
| LVESV/BSA (ml/m2) | 24.42±12.54 | 25.16±9.87 | 0.819 |
| LVEDV/BSA (ml/m2) | 55.85±23.14 | 54.53±15.05 | 0.814 |
| LV GLS (%) | -13.55±1.82 | -16.11±1.33 | <0.001 |
| LV GCS (%) | -25.81±7.57 | -26.11±5.92 | 0.879 |
| LV GRS (%) | 26.36±11.61 | 32.43±10.50 | 0.058 |
| LV AT (%) | -35.58±9.02 | -39.27±5.81 | 0.092 |
| LV twist (°) | 4.80±2.59 | 4.79±2.83 | 0.992 |
| Multivariate adjustment | |||
| LA LS | - | - | 0.026 |
| LV GLS | - | - | <0.001 |
Values are mean ± standard deviation.
2D: two-dimensional; 3D: three-dimensional; AT: area tracking; BSA: body surface area; DM1: myotonic dystrophy type 1; dT: deceleration time; GCS: global circumferential strain; GLS: global longitudinal strain; GRS: global radial strain; IVS: intraventricular septum; LA: left atrial; LAEF: left atrial emptying fraction; LAEDV: left atrial end-diastolic volume; LAESV: left atrial end-systolic volume; LS: longitudinal strain; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; PW: posterior wall; TAPSE: tricuspid annular plane systolic excursion.
Also, three-dimensional echocardiography did not show significant differences in LVEF (62.88%±3.27 vs. 65.64%±6.65, p=0.80), LV mass index (91.83 g/m2±21.51 vs. 85.80 g/m2±15.73, p=0.267), LV end-systolic (24.42 ml/m2±12.54 vs. 25.16 ml/m2±9.87, p=0.819) or end-diastolic volume (55.85 ml/m2±23.14 vs. 54.53 ml/m2±15.05, p=0.814), or in LA emptying fraction (42.13%±8.53 vs. 46.37%±11.21, p=0.139) or indexed end-systolic (13.12 ml/m2±4.35 vs. 13.12 ml/m2±3.50, p=0.569) and end-diastolic volumes (22.70 ml/m2±4.75 vs. 23.60 ml/m2±6.19, p=0.677).
The percentage of segments analyzed with 3D-STE was 86.25%.
Patients with DM1 showed significantly lower LA LS than controls (22.85%±5.06 vs. 26.82%±5.15; p=0.008), which was not the case with AT (21.77%±17.49 vs. 25.41%±12.94; p=0.412). There was a significant correlation between age and LA LS in patients with DM1 (r=-0.771; p<0.001; Table 3).
Correlations in patients with myotonic dystrophy type 1.
| r | p | |
|---|---|---|
| PR interval – LA LS | -0.304 | 0.192 |
| Age – LA LS | -0.771 | <0.001 |
| PR interval – LV GLS | 0.028 | 0.906 |
| QRS – LV GLS | 0.310 | 0.183 |
| LV mass – LV GLS | 0.368 | 0.077 |
GLS: global longitudinal strain; LA: left atrial; LS: longitudinal strain; LV: left ventricular; LV mass: left ventricular mass index on three-dimensional echocardiography.
LV GLS was significantly lower in patients with DM1 than in controls (-13.55%±1.82 vs. -16.11%±1.33; p<0.001), which was not the case for GCS (-25.81%±7.57 vs. -26.11%±5.92; p=0.879), GRS (26.36%±11.61 vs. 32.43%±10.50; p=0.058), AT (-35.58%±9.02 vs. -39.27%±5.81; p=0.092) or twist (4.80±2.59 vs. 4.79±2.83, p=0.992). No correlation was found between PR interval (r=0.028; p=0.906), QRS (r=0.310; p=0.183) or 3D LV mass index (r=0.368; p=0.077) and LV GLS in DM1 patients (Table 3).
Multivariate adjustment confirmed that LA LS and LV GLS remained statistically significant (p=0.026 and p<0.001, respectively).
Interobserver and intraobserver agreement for strain measurements was good. The ICCs are presented in Table 4.
Interobserver and intraobserver agreement.
| Intraobserver agreement | Interobserver agreement | |
|---|---|---|
| LA LS | 0.80 | 0.92 |
| LA AT | 0.89 | 0.83 |
| LV GLS | 0.95 | 0.91 |
| LV GCS | 0.83 | 0.90 |
| LV GRS | 0.90 | 0.89 |
| LV AT | 0.86 | 0.96 |
| LV twist | 0.87 | 0.91 |
Values are means of intraclass correlation coefficient.
AT: area tracking; GCS: global circumferential strain; GLS: global longitudinal strain; GRS: global radial strain; LA: left atrial; LV: left ventricular.
The assessment of myocardial deformation has been proposed as an early marker of subclinical dysfunction in DM1 patients.2 The present study is, to our knowledge, the first to identify changes in both LA and LV strain measured by 3D-STE in these patients.
LA mechanics is considered an important clinical variable and its importance has been demonstrated in a number of conditions.13–19 LA strain curves display the physiology of atrial function. The left atrium has four basic functions: phase 1, reservoir function (collection of pulmonary venous flow during LV systole); phase 2, conduit function (passage of blood to the left ventricle during early diastole); phase 3, active contractile pump function; and phase 4, suction force.20 During the reservoir phase, the left atrium is stretched, and consequently longitudinal atrial strain increases, reaching a positive peak at the end of atrial filling. After the atrium empties, LA strain decreases to a plateau, corresponding to LA diastasis, and then continues to decrease during the LA contraction phase (Figure 1).
In our study, a significant negative correlation was observed between age and LA LS in patients with DM1, which did not occur with the controls, suggesting a faster decrease in LA LS in these patients.
In DM1 patients, 2D-STE is able to identify changes in LS of the right atrium, which correlate with low-voltage areas on electrophysiological study.21 However, LA mechanics has been little studied and validated in these patients, so it is difficult to draw definite conclusions about these findings. We expect that with future larger studies, these changes may prove to be important for early diagnosis and therapeutic decision-making.
Studies with 2D echocardiography are consistent in demonstrating decreased LV GLS in these patients,9,22 as well as changes in GCS.9 In our study, we found significant differences only in GLS, which may mean that these fibers are affected early in the progression of the disease, subsequently being replaced by fibrosis, as demonstrated in previous studies23 and other cardiomyopathies, such as hypertrophic cardiomyopathy.24 The presence of myocardial fibrosis in these patients has been documented by a cardiovascular magnetic resonance study,23 in which myocardial fibrosis was found in 40% of patients and was not related to changes on electrocardiogram, Holter or echocardiogram. This suggests that assessment of myocardial fibrosis by cardiac magnetic resonance may be a useful tool in the assessment of these patients, since myocardial fibrosis may be the substrate for ventricular arrhythmias. Also, high levels of transforming growth factor beta 1, a sensitive marker of fibrosis, have been reported in patients with DM1, indicating a higher risk of arrhythmic events and sudden death.25
Previous studies have shown the usefulness of subclinical detection of cardiac involvement in DM1. In one study of 36 DM1 patients, serial Holter monitoring resulted in pacemaker implantation in 11 patients,26 and implantable loop recorders have been used in small studies of asymptomatic patients, leading to the detection of ventricular arrhythmias and device implantation in four out of seven patients.27,28 A recently published study demonstrated the prognostic value of LV GLS in patients with asymptomatic DM1, concluding that GLS constitutes a strong predictor of cardiovascular events.29
3D wall motion tracking uses a box template to detect speckle motion in 3D. The structures are captured in a full volume and do not move out of plane like in 2D. 3D-STE has also been shown to provide a faster and more complete assessment than 2D-STE, overcoming some of the shortcomings of previous techniques by reducing the intraobserver and interobserver variability inherent in 2D measures and avoiding the angle dependence of strain and strain-rate measures obtained on Doppler tissue imaging.7 Nesser et al.30 assessed the accuracy of measuring LV volumes by means of 3D-STE side by side with 2D-STE using cardiac magnetic resonance as a reference. They observed that measurements by 3D-STE showed higher correlation with cardiac magnetic resonance, smaller biases and narrower limits of agreement. 3D-STE is emerging as a useful tool for a more comprehensive assessment of ventricular function and atrial mechanics and may be valuable in a general and systematic approach to these patients, bearing in mind its possible prognostic impact.
This study has some limitations. One is the small number of patients (total 25), which is in line with the rarity of the disease (estimated prevalence of about 1/10000) and previous studies performed in patients with this condition. It also has the inherent limitations of an observational study and, as pointed out above, the clinical impact of LA deformation changes are still not well understood. Regarding image acquisition, a major limitation for current 3D-STE systems is their relatively low temporal resolution, with typical frame rates of 20-25 volumes/s, and sequential volume acquisition, which is vulnerable to motion artifacts if the patient is unable to cooperate. The software used continues to improve but is not fully validated for the study of the left atrium.
ConclusionLA LS and LV GLS are significantly decreased in patients with DM1. These results may reflect a subclinical and early marker of myocardial dysfunction in these patients. Studies with 3D-STE can detect changes in myocardial function that may contribute to the assessment and follow-up of these patients. Future prospective studies may open new perspectives on the clinical relevance of these markers.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors are grateful to Leopoldo Pérez de Isla, MD, and Fabián Islas, MD, both from the Cardiology Department, Hospital Clínico San Carlos, Madrid, Spain.







 GLS (E) are also presented; (3) LV
GLS (E) are also presented; (3) LV 

