Cardiovascular disease is the leading cause of mortality and morbidity associated with diabetes. Although impairment of the cell response to hypoxia due to destabilization of the transcription factor hypoxia-inducible factor-1α (HIF-1α), which regulates the expression of genes that help cells to cope with low oxygen tension, has been implicated in diabetes-associated disease, the molecular mechanisms involved remain elusive. It is known that hyperglycemia leads to the enhanced production of methylglyoxal (MGO). Therefore, the main objective of this study was to establish whether MGO leads to the degradation of HIF-1α in cardiomyocytes subjected to hypoxia.
MethodsThe mouse atrial cardiomyocyte cell line, HL-1, was exposed to chemical hypoxia with CoCl2 in the absence or presence of MGO. Cell viability was assessed by MTT assay, and levels of HIF-1α and endogenous ubiquitin conjugates were determined by western blotting. Proteasome activity was analyzed using a specific chymotrypsin-like fluorogenic substrate.
ResultsThe results obtained indicate that MGO induces time- and dose-dependent degradation of HIF-1α accumulated under hypoxia. Additionally, we show that accumulation of endogenous ubiquitin conjugates in the presence of MGO is associated with decreased proteasome activity.
ConclusionTaken together, the results obtained in this study suggest that MGO compromises the ability of cells to adapt to low oxygen tensions, by stimulating the degradation of HIF-1α, likely contributing to the development of diabetes-associated cardiac dysfunction.
As complicações cardiovasculares constituem a principal causa de morbimortalidade em doentes diabéticos. A destabilização do fator de transcrição induzido pela hipoxia (HIF-1α), que regula a expressão de genes de adaptação celular a condições de baixos níveis de oxigénio, parece comprometer a resposta celular de diversos tecidos à hipoxia, em condições de hiperglicemia. No entanto, os mecanismos moleculares subjacentes à destabilização do HIF-1α estão ainda por estabelecer. Está bem estabelecido que em situações de hiperglicemia ocorre o aumento da produção de metilglioxal (MGO). Assim, o objetivo deste trabalho foi estabelecer o impacto do MGO na degradação do HIF-1α em cardiomiócitos sujeitos a condições de hipoxia.
MétodosUma linha celular de cardiomiócitos da aurícula de ratos, HL-1, foi sujeita a hipoxia química com CoCl2, na ausência e na presença de MGO. De seguida, avaliou-se a viabilidade celular pelo ensaio de MTT, e determinaram-se os níveis de HIF-1α e de conjugados endógenos de ubiquitina por western blotting. A atividade do proteosoma foi avaliada utilizando um substrato fluorogénico específico do tipo quimotripsina.
ResultadosOs resultados obtidos mostram que o MGO induz degradação do HIF-1α em condições de hipoxia de uma forma dependente da dose de MGO utilizada, assim como do tempo de tratamento. Por outro lado, a acumulação de conjugados endógenos de ubiquitina na presença de MGO associa-se a um decréscimo na atividade do proteosoma.
ConclusãoNo conjunto, os resultados sugerem que o MGO compromete a capacidade de adaptação celular a condições de hipoxia através da degradação do HIF-1α, o que pode contribuir para o desenvolvimento da disfunção cardíaca associada à diabetes.
angiopoietin 2
chaperone-mediated autophagy
cobalt chloride
hypoxia-inducible factor
methylglyoxal
carbobenzoxy-L-leucyl-L-leucyl-L-leucinal
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
phosphate-buffered saline
Von Hippel-Lindau tumor suppressor protein
ubiquitin-proteasome pathway
vascular endothelial growth factor
One of the most important mechanisms involved in the response to oxygen restriction is that regulated by the transcription factor hypoxia-inducible factor-1 (HIF-1), a heterodimer formed of a stable nuclear subunit, HIF-1β, and a labile cytosolic subunit, HIF-1α. When exposed to low oxygen levels, cells mount a complex response that involves a myriad of mechanisms and signaling pathways that help cells to cope with low oxygen tensions. Under normoxia, HIF-1α undergoes hydroxylation of two proline residues (P402 and P564),1,2 catalyzed by specific prolyl hydroxylases. Hydroxylated HIF-1α is then recognized by von Hippel-Lindau tumor suppressor protein (pVHL), which is part of an ubiquitin ligase complex that promotes the ubiquitination and subsequent degradation of HIF-1α by the 26S proteasome,3,4 a multicatalytic proteolytic complex that recognizes and processes polyubiquitinated proteins through an ATP-dependent process.5–7 However, in hypoxic conditions, since HIF-1α is no longer hydroxylated, it accumulates and is translocated into the nucleus, where it dimerizes with the β subunit and initiates a genetic transcription program that includes synthesis of vascular endothelial growth factor (VEGF),8 which promotes the formation of new blood vessels.
Diabetes is one of the strongest risk factors for heart disease. Indeed, diabetic heart disease, including coronary heart disease, heart failure and diabetic cardiomyopathy, is the leading cause of death in people with diabetes. As with other cells, the cardiomyocyte response to ischemia involves increases in VEGF and angiopoietin 2 (Ang-2) levels that stimulate angiogenesis and myocardial perfusion. However, in diabetic patients, myocardial ischemia results in an increase in Ang-2 levels that is not accompanied by an increase in VEGF levels. In such conditions, Ang-2 exerts an anti-angiogenic effect, leading to programmed cell death of cardiomyocytes.9 Moreover, in diabetes, an inappropriate cell response to hypoxia has been associated with increased degradation of HIF-1α.10 Recent studies from our laboratory established that besides the ubiquitin-proteasome pathway (UPP), HIF-1α can also be degraded in the lysosome, through chaperone-mediated autophagy (CMA).11 However, the molecular mechanisms involved in the destabilization of HIF-1α in diabetes remain elusive. Some studies suggest that methylglyoxal (MGO), a highly reactive byproduct of glycolysis that accumulates in diabetic patients and has been implicated in several complications of the disease,12,13 promotes the degradation of HIF-1α with consequent reduction of VEGF expression in hypoxic conditions.2 These reactive carbonyl compounds form covalent adducts with particular lysine and arginine residues in proteins, altering their life cycle, including stability and function.14–16
The main goal of this study was to elucidate the mechanisms whereby diabetes contributes to cardiac dysfunction. Specifically, we aimed to assess how MGO affects the response of cardiomyocytes to hypoxia, particularly HIF-1α levels. We also assessed the role played by UPP in MGO-induced destabilization of HIF-1α.
MethodsCell culture and treatmentsHL-1 adult murine cardiomyocytes (kindly donated by Prof. W. Claycomb, Department of Biochemistry and Molecular Biology, LSUHSC, New Orleans, LA, USA) were cultured in Claycomb Medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mM glutamine, and 0.1 mM norepinephrine in an atmosphere of 95% O2 and 5% CO2 at 37°C. The HL-1 cells were grown under fibronectin/gelatin, and were treated with CoCl2, carbobenzoxyl-leucinyl-leucinyl-leucinal-H (MG132), and MGO (Sigma-Aldrich, St. Louis, MO, USA).
Cell viability assayThe viability of the HL-1 cells was assessed using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay. Briefly, after treatments, cells were incubated with MTT solution (0.5 mg/ml) for two hours at 37°C in a cell culture incubator. Subsequently, supernatants were removed and acidified isopropanol (0.04 N HCl in isopropanol) was added to dissolve the dark blue crystals of formazan produced by metabolically active cells. Formazan was quantified by measuring the absorbance of the samples using an ELISA automated microplate reader (Biotek, Winooski, VT, USA) at 570 nm, with a reference wavelength of 620 nm.
Western blotAfter the treatments, the cells were washed twice with phosphate-buffered saline (PBS) and denatured in a Laemmli buffer, sonicated and boiled at 95°C for 5 min. The denatured proteins were separated by polyacrylamide gel electrophoresis and electrophoretically transferred onto nitrocellulose membranes. The membranes were blocked for 1 h with 5% m/v of nonfat milk at room temperature and for 1 h with HIF-1α (1:500; SICGEN, Cantanhede, Portugal), β-actin (1:1000, Sigma Aldrich, St. Louis, MO, USA), GADPH (1:5000; SICGEN, Cantanhede, Portugal) and ubiquitin (1:1000, BioLegend, San Diego, CA, USA) primary antibodies at 4°C overnight, followed by incubation with the corresponding secondary antibodies at room temperature for 1 h. Specific protein bands were detected using an electrochemiluminescence detection system (Bio-Rad, USA). β-actin and GAPDH were used as loading control.
Chymotrypsin-like activityThe HL-1 cells were washed with PBS, lysed with 50 mM Tris, pH 7.6, supplemented with 1 mM DTT and sonicated. After centrifugation (16000 g for 10 minutes at 4°C), the protein concentration was determined and 40 μg of protein was incubated with 100 μM Suc-LLVY-MCA (Biomol-Enzo Life Sciences, Farmingdale, NY, USA) in a 96-well plate. Chymotrypsin-like activity was monitored for 1 h at 37°C in 5 min periods (excitation wavelength 380 nm; emission wavelength 460 nm). Absorbance was measured on a Biotek Synergy HT spectrophotometer (Biotek, Winooski, VT, USA).
ImmunoprecipitationCell lysates were prepared by solubilization in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.1% SDS, pH 7.6), containing protease and phosphatase inhibitors. The samples were centrifuged at 3200 rpm for 5 min, and the supernatants used for immunoprecipitation. Briefly, the supernatants were incubated with antibodies against HIF-1α. Non-specific (anti-GFP) antibodies were used for negative controls. Incubations proceeded overnight at 4°C followed by incubation with protein G-sepharose (Sigma-Aldrich, St. Louis, MO, USA) for 2 h at 4°C. The protein G-sepharose sediments were washed in RIPA buffer, eluted in Laemmli buffer, denatured at 95°C for 5 min, and analyzed by western blot.
Statistical analysisThe results are expressed as mean ± standard error of the mean of at least three independent experiments. The data were analyzed using one-way analysis of variance followed by the Tukey post-hoc test, using GraphPad Prism 5.0 software (San Diego, CA, USA). Differences between means were considered significant for values of p<0.05.
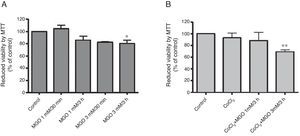
ResultsMethylglyoxal reduces cell viability in HL-1 cellsHyperglycemia was previously shown to be involved in the loss of cell response to hypoxia. In this study, a cardiac cell line (HL-1) was used to investigate the role of MGO in the regulation of HIF-1α, a transcription factor involved in the cell response to low oxygen tension. The results presented in Figure 1A show that MGO induces a reduction in cell viability in a dose-dependent manner. We then assessed the effect of MGO in cells under hypoxia. To subject the cells to conditions that partially mimic hypoxia, HL-1 cells were incubated with CoCl2. HL-1 cells treated with CoCl2 for 6 h in the presence of 3 mM of MGO added to the culture in the final 3 h present a significant reduction in cell viability compared to cells exposed to hypoxia in the absence of MGO (Figure 1B). Importantly, incubation with CoCl2 alone did not affect cell viability.
Cell viability under hypoxic conditions. HL-1 cells were subjected to MGO treatment in the absence (A) or presence of chemical hypoxia (B), and MTT assay was used to assess cell viability. The percentage of cytotoxicity was calculated relative to control (untreated cells). Data are mean ± standard error of the mean of three independent experiments performed in quadruplicate. * p<0.05, significantly different from control cells and MGO 1 mM/30 min; ** p<0.01, significantly different from control cells (one-way analysis of variance with Tukey's multiple comparison test). MGO: methylglyoxal.
Previous data from our group demonstrated that MGO induces the degradation of HIF-1α in retinal epithelial cells under hypoxia.2 However, the impact of MGO on HIF-1α in cardiac cells has never been addressed. We first investigated the effects of various MGO concentrations on HIF-1α levels in HL-1 cells under hypoxic conditions. After reaching confluence, the cells were exposed to CoCl2 to induce chemical hypoxia for 6 h and MGO (1 mM or 3 mM) was added in the last 30 min or 3 h of hypoxia. After the treatments, the cells were harvested and HIF-1α levels were determined by western blotting. As expected, under normoxic conditions HIF-1α was undetectable (Figure 2A, lane 1), but after 6 h of incubation with CoCl2 the amount of HIF-1α increased dramatically (Figure 2A, lane 2). However, when cells were simultaneously incubated with CoCl2 and MGO for 3 h, MGO impaired hypoxia-dependent accumulation of HIF-1α (Figure 2A; compare the 110 kDa band in lanes 4 and 6 with lane 2), suggesting that MGO induces degradation of HIF-1α accumulated in the course of hypoxia.
Effect of MGO on HIF-1α levels under hypoxia conditions. (A) HL-1 cells were exposed to CoCl2 (400 μM) for 6 h and then MGO (1 mM or 3 mM) was added in the last 30 min or 3 h. Whole-cell extracts were prepared and analyzed by western blotting using anti-HIF-1α or anti-β-actin antibodies (loading control); (B) HL-1 cells were exposed to CoCl2 (400 μM for 6 h) and MGO (3 mM for the last 3 h of treatment). HIF-1α was immunoprecipitated and immunoprecipitates were probed against HIF-1α and ubiquitin. MGO: methylglyoxal; WB: western blotting.
Interestingly, besides the presence of a 110 kDa band, under hypoxia HIF-1α accumulated as a slower migrating smear that most likely corresponded to ubiquitinated forms (Figure 2A). Strikingly, when HL-1 cells were simultaneously incubated with CoCl2 and MGO for 30 min, the intensity of the slower migrating bands was significantly increased, suggesting that ubiquitination was being enhanced (Figure 2A). However, for longer periods of incubation with MGO, reduction of both 110 kDa and high molecular weight bands of HIF-1α was observed (Figure 2A, lane 4 and lane 6 compared with lane 3 and lane 5), strongly suggesting that HIF-1α undergoes degradation in these conditions. To address whether MGO reduces the ubiquitination of HIF-1α, the protein was immunoprecipitated and probed with anti-ubiquitin antibodies. The results obtained show that levels of ubiquitinated HIF-1α increase in cells treated with MGO (Figure 2B, compare lane 1 with lane 2 of IP). We also assessed the effects on endogenous ubiquitin conjugates by western blot using polyclonal anti-ubiquitin antibodies (Figure 2B, Input). The results showed an increase in the endogenous conjugates of high molecular weight ubiquitin in cells subjected to hypoxia and in the presence of MGO.
Having established that MGO promotes degradation of HIF-1α under hypoxia, we proceeded to assess the involvement of UPP in the destabilization of HIF-1α. For this, HL-1 cells subjected to hypoxia in the presence of MGO were incubated with a proteasome inhibitor (MG132), after which HIF-1α levels were assessed. In these conditions an accumulation of HIF-1α was observed (Figure 3A, compare lane 3 with lane 5), suggesting that MGO induces HIF-1α degradation by the proteasome under hypoxia. Moreover, as expected, under normoxic conditions HIF-1α was undetectable. However, the results show an accumulation of HIF-1α when the cells were treated with a proteasome inhibitor in normoxia, confirming that HIF-1α is mainly degraded by the proteasome in normoxic conditions (Figure 3B, compare lane 1 with lane 2).
Effect of MGO on UPP activity. (A) HL-1 cells were treated with CoCl2 (400 μM; 6 h) with MGO (3 mM; 3 h) and MG132 (20 μM; 4 h). The cell extracts were used to measure chymotrypsin-like activity using the fluorogenic substrate Suc-LLVY-AMC. The degree of change in chymotrypsin-like activity was calculated relative to control (untreated) cells. The values in the graph correspond to measurements after 30 min of activity. Data are mean ± standard error of the mean of three independent experiments performed in quadruplicate; (B) HL-1 cells were exposed to CoCl2 (400 μM; 6 h), MGO (3 mM; 3 h) and MG132 (20 μM; 4 h). Whole-cell extracts were prepared and analyzed by western blotting using anti-HIF-1α or anti-β-actin antibodies (loading control). MGO: methylglyoxal; UPP: ubiquitin-proteasome pathway.
We also assessed the effect of hypoxia and MGO on chymotrypsin-like activity of the 20S proteasome by degradation of the Suc-LLVY-MCA fluorogenic peptide (Figure 3B). The cells were treated with chemical hypoxia for 6 h in the presence or absence of MGO added to the culture medium in the final 3 h of hypoxia. Incubation with MG132 was used as a positive control. The results presented in Figure 3B show that proteasome activity decreased by about 50% in cells subjected to hypoxia. Moreover, simultaneous incubation with MGO did not have additional effects on proteasome activity.
DiscussionDiabetes is closely associated with cardiovascular morbidity and mortality, but the specific molecular basis linking diabetes with increased vulnerability to cardiovascular injury remains poorly understood. It has been shown that increased production and accumulation of MGO are hallmarks of diabetes.17 One of the mechanisms whereby MGO contributes to cytotoxicity is through the modification of proteins involved in cell repair following an insult such as hypoxia,2,18 and MGO impairs protein quality control mechanisms in retinal epithelial cells.18 Similarly, in the present study, we demonstrated that in a cardiac cell line MGO induces a significant decrease in cell viability.
Moreover, diabetic complications are associated not only with hyperglycemia but also with hypoxia. The cellular response to hypoxia is a complex process that involves activation of HIF-1α, which eventually leads to increased transcription of genes that help cells to cope with low oxygen levels. It is well established, for various cell types and tissues, that in diabetic conditions the degradation of HIF-1α is increased in hypoxic conditions, thus compromising cells’ ability to adapt and survive.2,19–23 However, the effect of MGO on cardiac cells’ response to low oxygen tension is still unclear. In diabetes, the HIF-1α pathway is deregulated, most likely caused by hyperglycemia. For example, the influence of hyperglycemia on HIF-1α expression and activation in hypoxic conditions is well documented in vivo by the comparative study of biopsy material from chronic diabetic foot ulcers and chronic varicose ulcers.24 HIF-1α has also been shown to play a role in the pathogenesis of renal interstitial fibrosis, and there appears to be a correlation between intensity of HIF-1α expression and severity of kidney disease.25 Moreover, HIF-1α plays an important role in the pathogenesis of diabetic retinopathy and is implicated in disease progression, particularly in the early stages of this malady.21 In the heart, experiments in rats demonstrated that hyperglycemia is associated with increased myocardial infarct size and reduced production of the HIF-1α protein.19 Previous studies have also assessed the impact of hyperglycemia on HIF-1α gene transcription in hypoxic conditions and concluded that there are no changes in HIF-1α mRNA levels in this situation,26 ascribing an important role to degradation in the destabilization of HIF-1α in hypoxia. However, the mechanisms underlying this dysfunctional response are far from being clarified. It has been shown that hyperglycemia impairs proteasome function through the action of MGO.27 We demonstrate that both MGO and hypoxia lead to a decrease in proteasomal chymotrypsin-like activity. Moreover, it has been consistently shown that in diabetic conditions an inappropriate cell response to hypoxia is associated with increased degradation of HIF-1α2 by both UPP and CMA.11 Similarly, in this study we show that in cardiomyocytes proteasome inhibitors do not fully prevent MGO-induced HIF degradation, reinforcing the hypothesis that other proteolytic mechanisms account for HIF-1α degradation in cardiomyocytes incubated with MGO.
The present study shows for the first time that MGO promotes the degradation of HIF-1α in cardiomyocytes in hypoxic conditions. However, because proteasome inhibitors do not fully prevent HIF-1α degradation and MGO decreases proteasomal chymotrypsin-like activity, it seems reasonable to suggest that other degradation pathways are likely involved in HIF-1α degradation induced by MGO, possibly by CMA.
Although these results bring new insights into the molecular mechanisms whereby diabetes contributes to diabetic cardiomyopathy, extrapolating them to the heart needs to be confirmed with analysis of samples from animal models and from human diabetic patients.
ConclusionIdentifying the molecular mechanisms underlying cell responses to oxygen depletion and the defective pathways involved in diabetes is a valuable contribution to a better understanding of the nature of morbid diabetic complications and to the development of new therapeutic strategies directed at the underlying molecular chain of events, in order to reduce the burden of morbidity and mortality that characterizes the disease. Based on this study, we propose that hyperglycemia increases MGO concentrations, which decreases proteasomal activity and induces destabilization of HIF-1α under hypoxic conditions. This destabilization may involve degradation of HIF-1α. However, because proteasome inhibitors do not completely prevent HIF-1α degradation, CMA may be involved in MGO-induced HIF-1α degradation. These results bring new insights into the molecular mechanisms whereby diabetes contributes to diabetic cardiomyopathy.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by the Foundation for Science and Technology, Portugal: UID/NEU/04539/2013 and COMPETE - POCI-01-0145-FEDER-007440.







 MGO treatment in the absence (A) or presence of chemical hypoxia (B), and
MGO treatment in the absence (A) or presence of chemical hypoxia (B), and 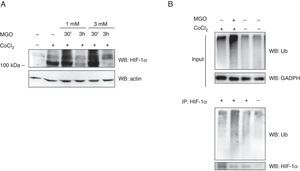 MGO on
MGO on 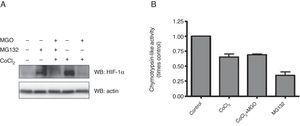 MGO on
MGO on 

