I read with great interest the single-center experience on spontaneous coronary artery dissection (SCAD) recently published by Abreu and colleagues in this journal.1 The authors reported a case series consisting of 27 patients, admitted to the cardiology department between January 2010 and December 2016, with a diagnosis of acute coronary syndrome (ACS) due to ongoing SCAD.
The prevalence of SCAD was 0.5% among patients who underwent catheterization for suspected ACS (15 NSTEMI, 10 STEMI and two sudden cardiac arrest). Most were women (22 F:5 M) and the cohort's median age was 56±11 years.
Patient characteristics and clinical presentation with predisposing factors were reported in Table 1 of the article. They were managed mainly in a conservative manner (15 medical therapy vs. 12 PCI). It is not insignificant that four of the 15 patients managed conservatively had a myocardial infarction on follow-up and in two of these the initial treatment was modified, requiring a switch to coronary angioplasty, with no cases of stent thrombosis at follow-up. A close clinical follow-up with optical coherence tomography (OCT) for PCI-managed cases was recommended. Prognosis was good despite the high prevalence of reinfarction in-hospital or during follow-up.
Considering the high level of interest in this subject, the European Society of Cardiology, in partnership with the Acute Cardiovascular Care Association, has established a European SCAD registry as a platform for collaborative research with the aim of improving awareness of the condition for better management.
Here I would like to put forward some food for thought that could be useful and interesting for the Journal's readers.
Of note, Abreu et al. remarked that SCAD is still an underestimated entity due to the challenging diagnosis, in which a high degree of clinical suspicion plays a key role. In this regard, I would like to underline that emerging evidence shows that this underestimation is mainly due to the absence of the classic angiographic hallmarks, which are lacking in >70% of angiographies2 and may be discovered only by intravascular imaging, namely OCT and intravascular ultrasound (IVUS).2,3
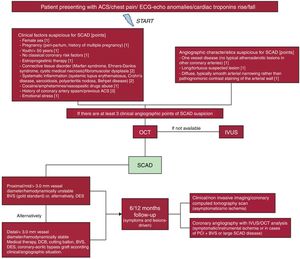
Considering the high rate of misdiagnosed SCAD,2–6 an interesting and useful score system (Figure 1) was previously published and tested6–8 allowing interventionists to select suspected cases in which intravascular imaging, particularly OCT, as the first choice, thanks to its higher spatial resolution (about 10 times greater than IVUS, which represents the second line) could identify the presence of SCAD, thus reducing the time to obtain the correct diagnosis and initiate appropriate therapy. The score is in the process of statistical validation on a larger cohort.
Flowchart for the diagnosis and management of spontaneous coronary artery dissection.7
Moreover, SCAD management remains challenging because of the lack of evidence supporting standard medical therapy, and the role of percutaneous or surgical revascularization is strongly debated.4,5,9 Abreu et al. state that, when necessary, a long stent or two stents were implanted, preventing the extension of intramural hematoma caused by stent compression against the vessel wall.
Conservative management (medical therapy) with aspirin, P2Y12 inhibitors, beta-blockers and statins is the preferred option according to a recently published experience-based survey. Alternatively, our group suggested invasive treatment with implantation of a drug-eluting stent or a bioresorbable scaffold (BRS) in cases of dissection involving vessels of ≥3 mm diameter or proximal vessel segments.7
In my opinion, and following our experience6–8,10,11 and the recent literature,12,13 these patients are eligible for bioresorbable scaffolding that allows vessel sealing, in consideration of the typical absence of atherosclerotic plaque rupture and the young age of most subjects affected, as in the cases reported, thus avoiding a permanent metal prosthesis.
In conclusion, our clinical-angiographic score could have helped provide the correct diagnosis, especially in challenging cases, thus allowing effective therapy that in my opinion should have been invasive, preferably with BRS implantation, considering the clinical presentation of ACS and for lesions longer than 3 mm or involving the proximal segment of coronary arteries, in view of the risk of potentially life-threatening complications that could have occurred in young people like the patients of the case series reported.
Conflicts of interestThe author has no conflicts of interest to declare.