We present first-in-human treatment with bioabsorbable magnesium scaffolds for percutaneous coronary intervention in a patient with nickel allergy. We present images from angiography and optical coherence tomography at three months. We also review the current status of these novel devices.
Apresentamos pela primeira vez o tratamento com suportes bioabsorbíveis de magnésio na intervenção coronária percutânea complexa de doentes com alergia ao níquel. Incluímos a angiografia e as imagens da tomografia de coerência óptica efetuadas durante um período de três meses. Analisamos também o estado atual deste equipamento inovador.
The Magmaris bioabsorbable sirolimus-releasing magnesium scaffold (Biotronik) is the first device of its kind that is clinically tested and with proven safety data. It features a metallic magnesium backbone with corrugated ring design, six crowns and strut thickness of 150×150 μm. In vitro tests have demonstrated greater radial strength, 20% less recoil and 80% more flexibility than the Absorb bioabsorbable sirolimus-releasing polylactic acid (PLLA) scaffold (Abbott).1
The magnesium backbone is coated with a compound called BIOlute, a resorbable PLLA polymer which delivers controlled release of the sirolimus drug for up to 90 days and which is metabolized in the body to carbon dioxide and water. The absorption process is rapid, with 95% of the magnesium being reabsorbed within twelve months.2
A safety study, BIOSOLVE I, enrolled 46 patients with 47 de novo lesions. Three-year follow-up results were obtained from 44 patients, which showed target lesion failure in three cases (6.6%), and no cardiac death or device thrombosis. Seven patients had additional angiographic follow-up at 28±4 months: in-scaffold late luminal loss had improved from 0.51±0.46 mm (median 0.28 mm) at 12 months to 0.32±0.32 mm (median 0.20 mm).3
The 12-month results of BIOSOLVE-II have recently been published, enrolling 123 patients with de novo lesions with a reference diameter between 2.2 mm and 3.7 mm. Quantitative coronary angiography parameters were stable from 6 to 12 months (paired data of 42 patients: in-segment late lumen loss 0.20±0.21 mm vs. 0.25±0.22 mm, p=0.117; in-scaffold late lumen loss 0.37±0.25 mm vs. 0.39±0.27 mm, p=0.446, respectively. Intravascular ultrasound demonstrated preserved minimal luminal area at six months (6.24±1.15 mm2 after percutaneous coronary intervention [PCI] vs. 6.21±1.22 mm2 at six months), with a small neointimal area (0.08±0.09 mm2). Optical coherence tomography (OCT) detected no intraluminal masses. Target lesion failure was observed in 3.4%, with no definite or probable scaffold thrombosis.4
Current recommendations for the Magmaris implant are de novo coronary lesions with a vessel diameter near the nominal size of the scaffold and a length shorter than the device. Among lesions not recommended or those in which special care has to be taken are total chronic occlusion, excessive tortuosity and side branches (bifurcations) greater than 2 mm in diameter.5
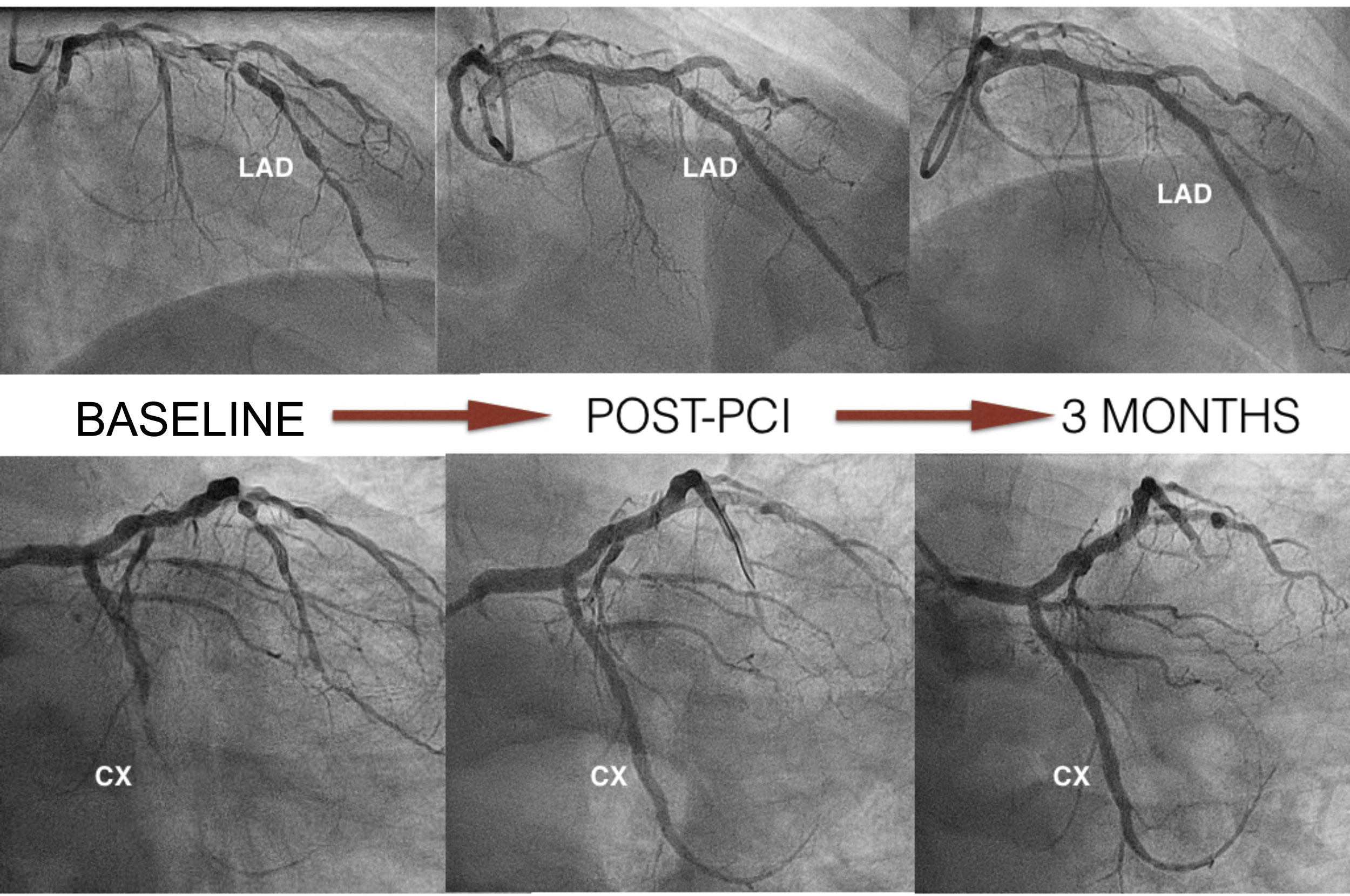
Case reportWe present the case of a 65-year-old male with nickel allergy who was admitted to the hospital with non-ST-elevation acute coronary syndrome. Coronary angiography showed significant two-vessel coronary disease not suitable for surgical revascularization because of small caliber and diffuse disease. There were multiple tandem lesions from the proximal to the distal left anterior descending (LAD) artery, bifurcated (Medina 1,1,1) with a diagonal branch, and a chronic total occlusion (CTO) in the mid circumflex (CX) (Figure 1).
The patient was on aspirin so a loading dose of 180 mg ticagrelor was added. For the coronary angiography 8000 U heparin (weight-adjusted) and 300 μg intracoronary nitroglycerine were administered, in order to obtain a better assessment of the actual size of the coronary arteries. No imaging techniques were performed because of difficulty in crossing the lesion.
The LAD was treated, first with predilatation with a 3.0 mm×20 mm non-complaint balloon in the distal LAD and the diagonal branch, and a 3.5 mm×20 mm balloon in the proximal LAD (balloon-scaffold ratio 1:1). Sequential and overlapping Magmaris scaffolds were then implanted (leaving 1 mm between the devices) from the distal LAD (one 3 mm×15 mm and two 3.5 mm×25 mm) to the bifurcation with the diagonal. StentBoost image enhancement (Philips) was used to adjust the overlap. Two more Magmaris scaffolds were then implanted in the median LAD (3.5 mm×25 mm) and the diagonal (3 mm×15 mm) by the reverse T and protrusion technique. The CTO of the CX was treated with a 3 mm×25 mm Magmaris. The scaffolds were implanted according to the manufacturer's recommendations to adequately predilate the lesion, select a device diameter very similar to vessel size, and postdilate without exceeding 0.6 mm above the nominal size of the device (Figure 1). In this case, we used non-compliant balloons with a 1:1 ratio except for the scaffold in the proximal LAD, which was postdilated with a 4.0 mm×20 mm non-compliant balloon. The final result was confirmed by OCT.
The patient was discharged with recommendation for 12 months dual antiplatelet therapy with aspirin 100 mg daily and ticagrelor 90 mg twice daily.
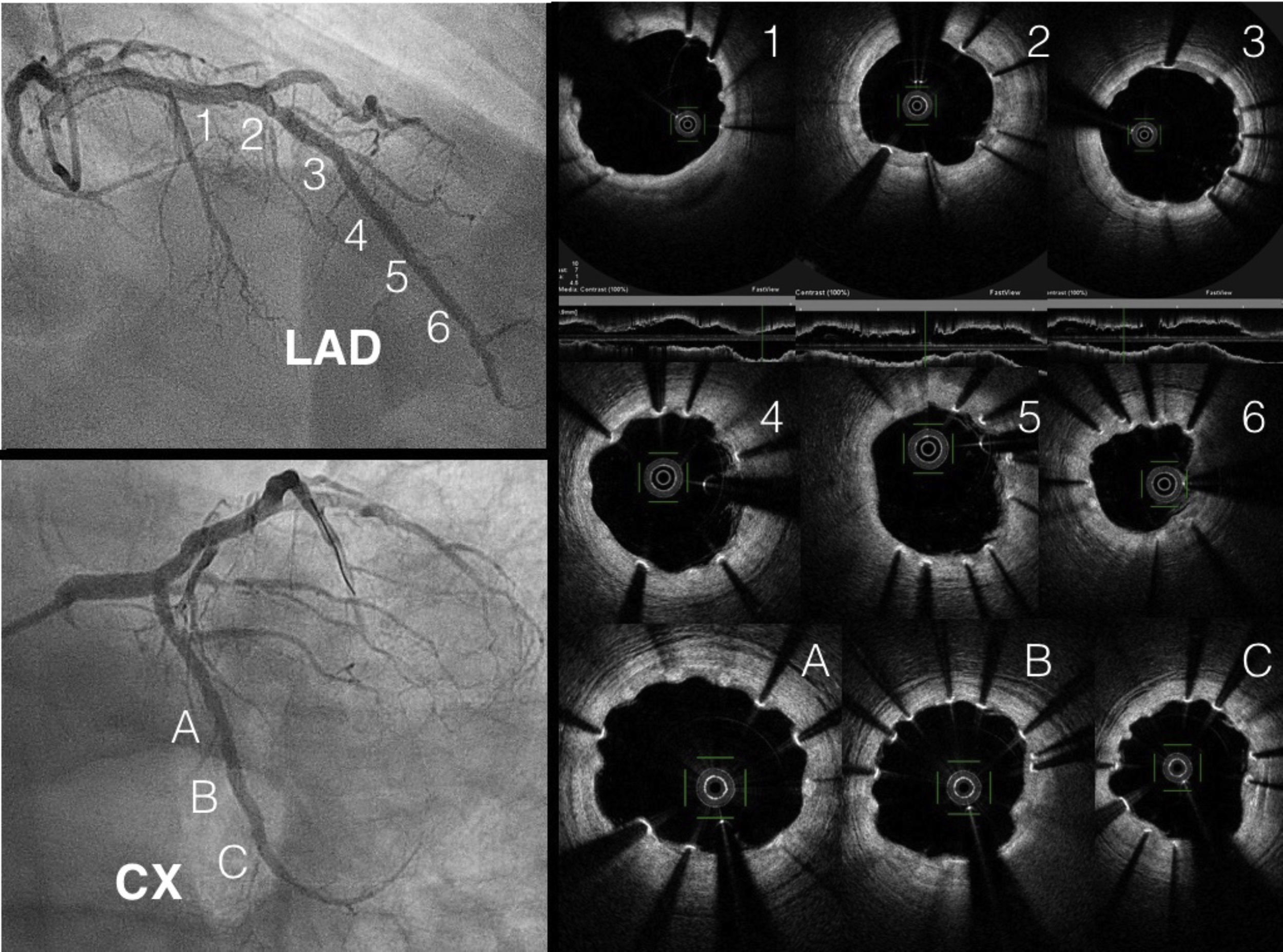
At three months, with no symptoms and with the patient's consent, coronary angiography was repeated (Figure 1). Three-month OCT showed appropriate strut apposition and partial covering of the majority of the struts (Figure 2).
ConclusionWe present first-in-human complex PCI in a patient with nickel allergy treated with Magmaris bioabsorbable sirolimus-releasing magnesium scaffolds (Biotronik). We highlight the good apposition in the CTO lesion and the true bifurcation and overlapping in the LAD, with good results on OCT. This novel device is becoming a real option in these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.