Coronary artery rupture is a rare but potentially fatal complication of percutaneous coronary intervention (PCI) that can result in life-threatening cardiac tamponade.
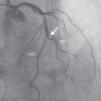
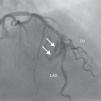
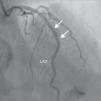
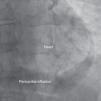
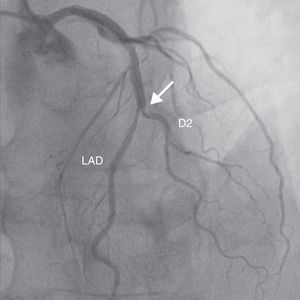
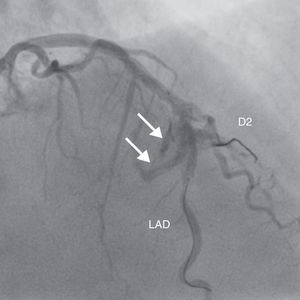
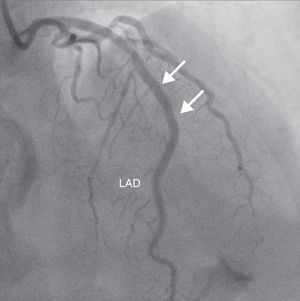
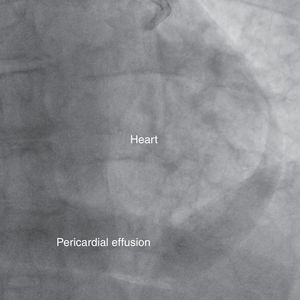
A 69-year-old man was referred for PCI of a 90% calcified lesion in the mid left anterior descending artery (LAD) involving the second diagonal branch (D2; Figure 1, arrow). After the left coronary ostium was cannulated and crossed with two BMW® wires, one to the LAD and other to the D2, predilation with a 2.5 mm×15 mm Trek® balloon was performed and an unsuccessful attempt was made to cross the stenosis with a 2.75 mm×22 mm Resolute Integrity® drug-eluting stent. Predilation was repeated with a 2.75 mm×15 mm Trek® non-compliant balloon at high pressure. Suddenly, balloon rupture was detected and the angiogram showed LAD rupture with extensive contrast extravasation into the pericardium (Figure 2, arrows; Video 1). Protamine sulfate was used to reverse the effect of heparin and the drug-eluting stent was deployed with balloon inflation for 10 minutes to seal the type III perforation but, as dye extravasation persisted, a 3.0 mm×19 mm GraftMaster® covered stent was superimposed, followed by rapid cessation of contrast leakage (Video 2). The D2 branch was lost (Figure 3, arrows), and periprocedural myocardial infarction occurred. The final image showed pericardial effusion (Figure 4; Video 3). The echocardiogram excluded tamponade. Forty-eight hours later, atrial fibrillation occurred with hemodynamic deterioration. Pericardiocentesis was performed and 50 ml of serosanguineous fluid was drained. Sinus rhythm was restored, with favorable evolution thereafter.
Treating calcified bifurcated lesions with balloons at high pressure must be performed with caution. If grade III perforation occurs, a standard stent can be used to save the side branch, although this is only successful in a minority of patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.