Sympathetic hyperactivity, a vital factor in the genesis and development of heart failure (HF), has been reported to be effectively reduced by percutaneous renal denervation (RDN), which may play an important role in HF treatment.
ObjectiveTo determine the effects of percutaneous RDN on cardiac function in patients with chronic HF (CHF).
MethodsFourteen patients (mean age 69.6 years; ejection fraction [EF] <45%) with CHF received bilateral RDN. Adverse cardiac events, blood pressure (BP), and biochemical parameters were assessed before and six months after percutaneous operation. Patients also underwent echocardiographic assessment of cardiac function and 6-min walk test before and at six months after percutaneous operation.
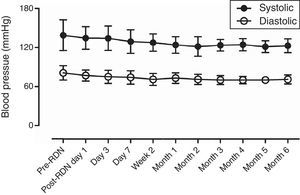
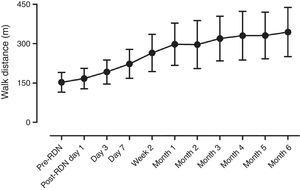
ResultsThe distance achieved by the 14 patients in the 6-min walk test increased significantly from 152.9±38.0 m before RDN to 334.3±94.4 m at six months after RDN (p<0.001), while EF increased from 36.0±4.1% to 43.8±7.9% (p=0.003) on echocardiography. No RDN-related complications were observed during the follow-up period. In 6-month follow-up, systolic BP decreased from 138.6±22.1 mmHg to 123.2±10.5 mmHg (p=0.026) and diastolic BP from 81.1±11.3 mmHg to 72.9±7.5 mmHg (p=0.032). Creatinine levels did not change significantly (1.3±0.65 mg/dl to 1.2±0.5 mg/dl, p=0.8856).
ConclusionRDN is potentially an effective technique for the treatment of severe HF that can significantly increase EF and improve exercise tolerance.
Foi reportado que a hiperatividade simpática, um fator vital para a génese e desenvolvimento da insuficiência cardíaca (IC), pode ser efetivamente reduzida pela desnervação simpática renal percutânea (DSR), que pode vir a desempenhar um papel importante no tratamento da IC.
ObjetivoDeterminar os efeitos da DSR por via percutânea na melhoria da função cardíaca em doentes com insuficiência cardíaca crónica (ICC).
MétodosCatorze doentes (idade média, 69,6 anos; fração de ejeção [FE], <45%) com ICC foram submetidos a RDN bilateral. Eventos cardíacos adversos, pressão arterial e índices bioquímicos foram realizados antes e seis meses após a intervenção. Os doentes também foram sujeitos a avaliação ecocardiográfica da função ventricular e ao teste de seis minutos de marcha, antes e seis meses após a intervenção.
ResultadosA distância percorrida pelos 14 doentes no teste de seis minutos de marcha aumentou de forma significativa, de 152,9 ± 38,0 m antes DSR para 334,3 ± 94,4 m, seis meses depois da DSR (p<0,001), enquanto a FE aumentou de 36,0 ± 4,1% para 43,8 ± 7,9% (p=0,003) no ecocardiograma. Não foram relatadas complicações da DRS durante o período de follow-up. Num follow-up de seis meses, a pressão sistólica desceu de 138,6 ± 22,1 mmHg para 123,2 ± 10,5 mmHg (p=0,026) e a pressão diastólica de 81,1 ± 11,3 mmHg para 72,9 ± 7,5 mmHg (p=0,032), sem alteração dos valores de creatinina (de 1,3 ± 0,65 mg/dl para 1,2 ± 0,5 mg/dl, p=0,8856).
ConclusãoA DSR é potencialmente uma nova e eficaz técnica para o tratamento da ICC severa, porque pode aumentar significativamente a FE e melhorar a tolerância ao exercício.
Chronic heart failure (CHF) affects about 100 million patients throughout the world, and has a five-year survival rate of only 50%, although drug therapy can improve symptoms. As an important factor in the genesis and development of heart failure (HF), sympathetic hyperactivity is directly correlated with the severity and prognosis of HF.1
Recently, it has been reported that a novel catheter-based renal sympathetic denervation (RDN) technique to ablate the renal afferent and efferent nerves2 can reduce excessive sympathetic nerve activation and may have a potential therapeutic effect in diseases associated with sympathetic activation.3 In some studies on hypertension, RDN has been found to lower blood pressure (BP) in patients with resistant hypertension, with a mean reduction of 33/12 mmHg.4,5 A previous study confirmed that RDN using a 3.5F Symplicity ablation catheter can extend the 6-min walk distance of patients with HF,6 but does not improve ejection fraction (EF) and left ventricular volume as shown by echocardiography. Our study group has demonstrated in preliminary animal experiments that percutaneous RDN using a 5F ablation catheter (Medtronic Inc., Dublin, Ireland) can improve cardiac function and reduce left ventricular volume in pigs with rapid pacing-induced HF.7 In the present study, a 5F ablation catheter was used for RDN in patients with CHF, and safety parameters such as cardiac function, exercise tolerance, BP, and creatinine (Cr) were assessed.
MethodsStudy populationInclusion criteria were a diagnosis of CHF; EF <45% on echocardiography; >2 HF episodes in the previous six months; medication with HF drugs including beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blocker (ARBs) and spironolactone; and no acute HF decompensation in at least one month of drug therapy.
Exclusion criteria were a history or evidence on imaging of renal artery stenosis (RAS); glomerular filtration rate <30 ml/min/1.73 m2; type 1 diabetes; pregnancy or intended pregnancy during the study period; acute phase of myocardial infarction (MI) or cerebrovascular accident; and systolic BP <100 mmHg.
Fourteen patients including four with hypertensive HF, two with dilated cardiomyopathy and eight with coronary artery disease were enrolled in the study. Mean baseline BP was 138.6±22.1/81.1±11.3 mmHg. All patients signed written informed consent forms for undergoing percutaneous RDN. The study was approved by the Ethics Committee of Putuo Hospital, Shanghai University of Traditional Chinese Medicine, China.
Trial designThis was a prospective, open, single-arm study without a control or sham group. All included patients received bilateral percutaneous RDN.
Pre-renal denervation assessmentAll included patients were assessed for BP, New York Heart Association functional class, echocardiographic parameters, 6-min walk distance, and routine blood biochemistry parameters (Tables 1–3). Bilateral renal artery ultrasound or computed tomography angiography was performed to exclude RAS before percutaneous RDN.
Characteristics of the study population.
| Age (years) | 69.6±5.7 |
| Male | 12/14 |
| Body weight (kg) | 76.7±5.0 |
| Height (cm) | 172.5±5.3 |
| NYHA functional class | |
| I | 0 |
| II | 0 |
| III | 10 |
| IV | 4 |
| Etiology | |
| Hypertension | 4 |
| CAD | 8 |
| DCM | 2 |
| CRT biventricular pacemaker | 1 |
| Automated ICD | 2 |
CAD: coronary artery disease; CRT: cardiac resynchronization therapy; DCM: dilated cardiomyopathy; ICD: implantable cardioverter-defibrillator; NYHA: New York Heart Association.
Comparison of echocardiographic parameters before and six months after renal denervation.
| Parameter | Baseline | 6 months | p |
|---|---|---|---|
| All patients | |||
| EF (%) | 36.0±4.1 | 43.8±7.9 | 0.002987 |
| LV end-diastolic diameter (mm) | 63.8±8.2 | 58.6±8.4 | 0.113991 |
| LV end-systolic diameter (mm) | 43.9±8.9 | 40.1±8.1 | 0.260472 |
| LA diameter (mm) | 43.9±5.2 | 41.9±5.7 | 0.342247 |
| Ventricular septal thickness (mm) | 10.8±2.2 | 10.6±1.3 | 0.83844 |
| Hypertensive patients | |||
| EF (%) | 34.5±4.3 | 52.3±6.1 | 0.004159 |
| LV end-diastolic diameter (mm) | 65.5±10.2 | 52.3±5.6 | 0.088789 |
| LV end-systolic diameter (mm) | 44.5±9.2 | 34.5±6.0 | 0.152822 |
| LA diameter (mm) | 42.3±4.1 | 42.5±7.9 | 0.958732 |
| Ventricular septal thickness (mm) | 11.6±2.9 | 11.5±1.9 | 0.90011 |
| Non-hypertensive patients | |||
| EF (%) | 36.6±3.8 | 41.0±6.2 | 0.072413 |
| LV end-diastolic diameter (mm) | 63.1±7.0 | 60.7±8.0 | 0.486515 |
| LV end-systolic diameter (mm) | 43.6±8.8 | 41.1±8.6 | 0.527247 |
| LA diameter (mm) | 44.6±5.5 | 41.7±5.1 | 0.235519 |
| Ventricular septal thickness (mm) | 10.4±1.7 | 10.3±0.9 | 0.873487 |
EF: ejection fraction; LA: left atrial; LV: left ventricular.
Comparison of biochemical and physiological parameters before and six months after renal denervation.
| Parameter | Baseline | 6 months | p |
|---|---|---|---|
| All patients | |||
| HR (bpm) | 64.8±4.3 | 60.9±4.0 | 0.018656 |
| Systolic BP | 138.6±22.1 | 123.2±10.5 | 0.026458 |
| Diastolic BP | 81.1±11.3 | 72.9±7.5 | 0.032108 |
| 6-min walk distance (m) | 152.9±38.0 | 334.3±94.4 | <0.001 |
| NYHA functional class | <0.001 | ||
| I | 0 | 4 | |
| II | 0 | 9 | |
| III | 10 | 1 | |
| IV | 4 | 0 | |
| Hypertensive patients | |||
| HR (bpm) | 68.2±4.5 | 64.5±2.6 | 0.200819 |
| Systolic BP | 167.5±17.1 | 126.3±12.5 | 0.008001 |
| Diastolic BP | 96.3±7.5 | 70.0±7.1 | 0.002236 |
| 6-min walk distance (m) | 157.5±20.6 | 370.0±128.3 | 0.01703344 |
| Non-hypertensive patients | |||
| HR (bpm) | 63.4±3.5 | 59.4±3.5 | 0.020427 |
| Systolic BP | 127.0±9.2 | 122.0±10.1 | 0.214079 |
| Diastolic BP | 75.0±4.7 | 74.0±7.7 | 0.7039 |
| 6-min walk distance (m) | 151.0±43.8 | 320.0±81.2 | <0.001 |
| Biochemistry | |||
| BNP (pg/ml) | 661.2±368.2 | 300.0±249.3 | 0.007935 |
| Cr (mg/dl) | 1.3±0.6 | 1.2±0.5 | 0.885653 |
| BUN (mg/dl) | 24.7±9.3 | 23.6±4.5 | 0.50941 |
| UA (mg/dl) | 6.9±1.6 | 6.6±1.7 | 0.637088 |
| Blood glucose (mg/dl) | 124.3±41.4 | 120.7±27.0 | 0.835591 |
| Blood sodium (mg/dl) | 320.7±9.4 | 321.8±7.6 | 0.734254 |
| Blood potassium (mg/dl) | 16.0±1.6 | 16.0±1.6 | 0.898615 |
BNP: brain natriuretic peptide; BP: blood pressure; BUN: blood urea nitrogen; Cr: creatinine; HR: heart rate; NYHA: New York Heart Association; UA: uric acid.
The RDN group was provided chewable enteric-coated aspirin 300 mg or clopidogrel 300 mg before the intervention and intravenous unfractionated heparin 6000∼8000 U was administered. Following skin preparation and disinfection of the right inguinal region, the right femoral artery was punctured and a 7F catheter sheath was inserted, followed by a JR catheter for bilateral renal angiography. A 5F radiofrequency catheter was subsequently inserted for rotational ablation using a 39D72X Stockert EP Shuttle RF Generator (Johnson & Johnson Medical) with temperature control (8-10 W, 50°C). The effective ablation time per point was 60-90 s (impedance reduction >10%) and 4-6 ablation points were applied to each renal artery (distance between neighboring points 0.5 cm). Renal angiography was performed after the percutaneous intervention.
Post-renal denervation assessmentAll patients remained in hospital for seven days for observation after the procedure. BP and 6-min walk distance were measured on the seventh day. At six-month follow-up after discharge, echocardiography, 6-min walk distance, and routine blood tests were performed, and BP and 6-min walk distance were monitored in all patients every month.
MedicationAll patients received the maximum tolerated dose of HF medications including beta-blockers, ACEIs, ARBs, and spironolactone. During the follow-up period, drug dosage was adjusted depending on patients’ BP, heart rate, and other parameters.
Statistical analysisThe statistical analysis was performed using SPSS 17.0. All data were expressed as mean ± standard deviation and compared between pre- and post-RDN using the t test, with statistically significant differences defined as p<0.05.
ResultsAll fourteen patients underwent bilateral RDN and completed six-month follow-up.
Safety of renal denervation for treating heart failureAfter bilateral RDN, neither hypotension nor syncope was observed during six-month follow-up, and renal function and Cr levels remained stable. Mean BP was 123.2±10.5/72.9±7.5 mmHg (significantly different from that of pre-RDN for all fourteen patients) (Table 3, Figure 1).
Assessment of symptoms and six-min walk test distanceDuring the follow-up period, all patients showed significantly improvement in HF symptoms such as chest distress and shortness of breath, and the number of hospitalizations due to HF was also significantly reduced (Table 3). The 6-min walk distance increased significantly, from 152.9±38.0 m before RDN to 334.3±94.4 m six months after RDN (p<0.001) (Figure 2).
Echocardiographic assessmentAll fourteen patients underwent echocardiographic assessment at six-month follow-up, when the echocardiographic parameters were EF 43.8±7.9%, left ventricular (LV) end-systolic diameter 40.1±8.1 mm and LV end-diastolic diameter 58.6±8.4 mm. There was a significant difference between pre- and post-RDN EF (p=0.003).
In particular, the four patients with hypertensive HF showed significantly improved echocardiographic parameters after RDN compared with pre-RDN (mean EF: 52.3±6.1% vs. 34.5±4.3%; LV end-systolic diameter: 34.5±6.0 mm vs. 44.5±9.2 mm; LV end-diastolic diameter: 52.3±5.6 mm vs. 65.5±10.2 mm). Mean BP was 126.3±12.5/70.0±7.1 mmHg, significantly different from that of pre-RDN (167.5±17.1/96.3±7.5 mmHg) for all four hypertensive patients (Table 3).
Changes in heart failure medications following renal denervationSome changes in HF medications were observed at six months after RDN compared with before RDN. Six patients reduced or discontinued use of loop diuretics due to the disappearance of peripheral edema and the absence of HF symptoms such as chest distress and shortness of breath. No patient increased use of loop diuretics. The dosage of beta-blockers was reduced in six patients and increased in one; the dosage of ARBs was reduced in six patients, and increased in one (Table 4).
Changes to heart failure medication following renal denervation.
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| ACEIs | ||||||||||||||
| Perindopril | 8 mg | 4 mg | 4 mg | 8 mg | ||||||||||
| Benazepril | 10 mg | 5 mg | 10 mg | 10 mg | ||||||||||
| ARBs | ||||||||||||||
| Losartan | 100 mg | 50 mg | ||||||||||||
| Valsartan | 160 mg | 80 mg | ||||||||||||
| Irbesartan | 150 mg | 150 mg | ||||||||||||
| Loop diuretics | ||||||||||||||
| Furosemide | 80 mg | 40 mg | 20 mg | - | 40 mg | 20 mg | 40 mg | 40 mg | ||||||
| Torasemide | 5 mg | 5 mg | 5 mg | - | ||||||||||
| Spironolactone | 40 mg | 40 mg | 20 mg | - | 20 mg | 20 mg | 20 mg | 20 mg | 20 mg | 20 mg | 20 mg | - | ||
| Beta-blocker | ||||||||||||||
| Bisoprolol | 10 mg | 5 mg | 5 mg | 5 mg | 2.5 mg | 5 mg | 2.5 mg | 2.5 mg | 1.25 mg | 1.25 mg | 7.5 mg | 5 mg | 5 mg | 2.5 mg |
| Digoxin | 1.25 mg | 1.25 mg | ||||||||||||
| Calcium antagonist | ||||||||||||||
| Amlodipine | 5 mg | 5 mg | 5 mg | 5 mg | ||||||||||
| 8 | 9 | 10 | 11 | 12 | 13 | 14 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| 8 mg | 4 mg | 4 mg | 4 mg | ||||||||||
| 100 mg | 100 mg | 50 mg | 50 mg | ||||||||||
| 160 mg | 80 mg | 100 mg | 100 mg | ||||||||||
| 300 mg | 150 mg | ||||||||||||
| 40 mg | 40 mg | 20 mg | 20 mg | 20 mg | 20 mg | 40 mg | 20 mg | 40 mg | 20 mg | ||||
| 5 mg | 5 mg | 5 mg | -5 mg | ||||||||||
| 40 mg | 40 mg | 20 mg | 20 mg | 20 mg | 20 mg | 20 mg | 20 mg | 20 mg | 20 mg | - | |||
| e | |||||||||||||
| 10 mg | 10 mg | 5 mg | 2.5 mg | 2.5 mg | 2.5 mg | 2.5 mg | 2.5 mg | 5 mg | 5 mg | 7.5 mg | 5 mg | 5 mg | 2.5 mg |
| 1.25 mg | 1.25 mg | ||||||||||||
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; Post: after renal denervation; Pre: before renal denervation.
In 2009 and 2010, a series of trials demonstrated that RDN is likely to be an effective therapeutic method for treating resistant hypertension.4,5 However, the more recent HTN-3 trial,8 frustratingly, showed that RDN had a similar BP-lowering effect to that in the sham group, leading to widespread concern about the effectiveness of RDN. However, this has not spelled the end of studies on RDN, especially those on its effects in HF.6,9,10 RDN can block the renal sympathetic nerves, inhibiting activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS), and is thus regarded as a possible approach for the treatment of HF.
During the follow-up period in our study, all fourteen patients had increased EF on echocardiography. Results of the 6-min walk test indicated significant improvements in cardiac function and exercise tolerance after RDN. The dosage of loop diuretics was also reduced due to improved control of symptoms and signs of HF.
The results of this study showed that there were no complications such as RAS or renal artery dissection in these HF patients following bilateral RDN. During the six-month follow-up period, hypotensive events were not observed and renal function did not change significantly.
The Renal Artery Denervation in Chronic Heart Failure (REACH-Pilot) trial in seven patients with HF was another study on the effects of percutaneous RDN. The trial indicated that RDN improved 6-min walk distance and exercise tolerance, consistent with the results of the present study. However, the present study also demonstrated significant improvements in EF and left ventricular volume on echocardiography, unlike the REACH-Pilot study. This discrepancy is likely to be related to the ablation catheter used and to differences in procedures. A 5F ablation catheter was used in the present study. Compared with the 3.5F Symplicity catheter (Medtronic Inc.), a 5F catheter makes more contact with blood vessels and the radiofrequency energy more easily penetrates the vessel wall to ablate nerve plexuses in the adventitia. Additionally, thermal energy is more likely to accumulate and cause vascular stenosis when a 5F ablation catheter is used compared with smaller sizes.
HF of both hypertensive and nonhypertensive etiology was included in this study. The results demonstrated that RDN is more effective in the improvement of cardiac function (especially EF) in patients with hypertensive HF, probably due to the BP-lowering effect of RDN, indicating that RDN may most benefit HF patients with hypertensive heart disease.
Numerous studies have explored the relationship between RDN and HF. Nozawa et al.11 reported that RDN performed before MI significantly reduced ventricular filling pressure and improved cardiac function in rats. More recently, Clayton et al.12 confirmed in a rapid pacing-induced rabbit HF model the dependence of angiotensin receptor expression on sympathetic nerve activity. They also observed an increase in the expression of angiotensin II type 1 receptor in renal cortical vessels and a decrease in the expression of angiotensin II type 2 receptor in HF rabbits, both of which were diminished by renal denervation. Similarly, Schiller et al.13 reported the inhibition of sympathetic nerve activity by unilateral RDN in a rabbit model of rapid pacing-induced HF, which was beneficial for cardiac sympathovagal balance in HF rabbits. All these results support the idea that RDN can inhibit activation of the sympathetic system and the RAAS to improve cardiac function.
The 2016 European Society of Cardiology Congress made it clear that despite the disappointing results of HTN-3, research into RDN will continue14. The clinical efficacy of RDN in the treatment of heart failure is supported by some ongoing studies.15
The results of the present study indicate that RDN using a 5F ablation catheter can improve cardiac function, reduce HF symptoms and signs, and enhance exercise tolerance in patients with HF. But a large-scale, long-term, randomized controlled trial is needed to confirm the results, because the present study was only a clinical observation with a small sample and a short follow-up period (six months).
LimitationsThis was a single-center, non-blinded, non-randomized, non-controlled clinical study with a small patient sample and without exclusion of possible biases.
ConclusionsRDN is a promising new technique for the treatment of severe HF, but more long-term research and clinical evidence are needed. It can effectively increase EF on echocardiography and improve exercise tolerance. Its effects are especially clear in hypertensive patients, which may be due to its antihypertensive effects.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThe study was funded by the National Natural Science Foundation of China Youth Project (81303145), Shanghai Municipal Commission of Health and Family Planning Key Project (20134003), Shanghai Municipal Commission of Health and Family Planning General Program (201440492), and the Putuo District Shanghai independent innovation project (KW12201).
Conflicts of interestThe authors have no conflicts of interest to declare.