Pulse pressure (PP) is the difference between systolic and diastolic blood pressure, and is an independent predictor of atrial fibrillation (AF). In this study we investigated the relationship between PP and atrial conduction times.
MethodsThe study included 157 patients with essential hypertension. PP of 60 mmHg or more was regarded as elevated (n=56). Atrial electromechanical delay (EMD) was assessed with tissue Doppler echocardiography and P-wave dispersion (Pd) was calculated from the electrocardiogram.
ResultsLeft atrial volume index (23.6±4.9 ml/m2 vs. 25.2±6.5 ml/m2, p=0.141), left ventricular mass index (77.3±13.5 g/m2 vs. 80.9±19.6 g/m2, p=0.180) and grade I diastolic dysfunction (42% vs. 53%, p=0.242) were similar between groups. Inter-atrial (33.6±9.2 ms vs. 41.5±11.3 ms, p<0.001), intra-left atrial (23.0±8.8 ms vs. 28.2±10.6 ms, p=0.001) and intra-right atrial (10.5±5.8 ms vs. 13.2±4.9 ms, p=0.004) EMD were found to be higher in patients with elevated PP. P-maximum (108±8 ms vs. 114±9 ms, p<0.001) and Pd (30±13 ms vs. 38±13 ms, p<0.001) were also prolonged in patients with elevated PP. Multivariate linear regression analysis revealed that PP was independently associated with inter-atrial EMD (β=0.379, t=4.088, p<0.001).
ConclusionThis study showed that elevated PP is associated with prolonged atrial EMD and Pd. Atrial conduction is disturbed in hypertensive patients with elevated PP before the development of significant structural remodeling.
A pressão do pulso (PP) é a diferença entre as pressões sistólica e diastólica, e é um preditor independente da fibrilhação auricular. Neste estudo, investigamos a relação entre a PP e os tempos de condução auricular.
MétodosO estudo incluiu 157 doentes com hipertensão essencial. Uma PP de 60 mmHg ou superior foi considerada elevada (n=56). O atraso eletromecânico auricular foi avaliado através de ecocardiografia Doppler tecidular e a dispersão da onda P foi calculada através de eletrocardiograma.
ResultadosO índice do volume auricular esquerdo (23,6±4,9 mL/m2versus 25,2±6,5 mL/m2, p=0,141), o índice da massa ventricular esquerda (77,3±13,5 g/m2versus 80,9±19,6 g/m2, p=0,180) e a relação da disfunção diastólica grau I (42 versus 53%, p=0,242) foram semelhantes entre os grupos. Um atraso eletromecânico auricular inter (33,6±9,2 ms versus 41,5±11,3 ms, p<0,001), intraesquerdo (23,0±8,8 ms versus 28,2±10,6 ms, p=0,001) e intradireito (10,5±5,8 ms versus 13,2±4,9 ms, p=0,004) foram considerados superiores nos doentes com PP elevada. Uma pressão máxima (108±8 ms versus 114±9 ms, p<0,001) e uma dispersão da onda P (30±13 ms versus 38±13 ms, p<0,001) foram também prolongados nos doentes com PP elevada. Uma análise multivariada de regressão linear revelou que a PP estava independentemente associada ao atraso eletromecânico auricular (β=0,379, t=4,088, p<0,001).
ConclusãoEste estudo mostrou que a PP elevada está associada ao prolongamento do atraso eletromecânico auricular e da dispersão da onda P. A condução auricular revela mais alterações nos doentes hipertensos com PP elevada antes do desenvolvimento de remodelagem estrutural significativa.
Hypertension plays a significant role in the etiology of various cardiovascular diseases. Pulse pressure (PP), defined as the difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP), has a strong and independent association with cardiovascular morbidity and mortality.1
Atrial fibrillation (AF) is the most common sustained arrhythmia, for which the classical risk factors are age, diabetes, obesity, hypertension, left ventricular hypertrophy, coronary artery disease, heart failure and valvular disease.2 Previous studies have indicated that both high blood pressure (BP)3 and elevated PP independently predict development of AF.4 Additionally, increased PP is associated with left atrial (LA) enlargement, which is another known risk factor for AF.5 Although pathophysiologically it has been proposed that increased arterial stiffness can lead to increased left ventricular load by elevating PP, it has been determined that PP elevation is a more important risk factor for AF than increased arterial stiffness.6
Delayed atrial conduction is important in the development in AF via initiation of reentry.7 Atrial electromechanical delay (EMD), defined as the time from the onset of the P wave on the electrocardiogram (ECG) to the onset of the late diastolic wave (A) from the ventricular annulus and atrial walls on tissue Doppler imaging (the PA interval), is one of the indicators of an arrhythmogenic substrate in patients with AF.8,9
There are few data on the role of delayed atrial conduction in the development of AF in hypertensive patients with increased PP. In this study, we investigated the relationship between PP and atrial EMD, which are both important precursors of AF, in hypertensive patients with no structural cardiac abnormalities.
MethodsStudy populationThe study included 284 consecutive patients previously diagnosed with essential hypertension, aged 18-75 years. Detailed physical examinations were performed and medical histories were obtained from all patients. Complete blood count and biochemical analysis together with anthropometric measurements were performed on the same day as the echocardiographic examination. Antihypertensive treatments were recorded.
The following patients were not included in the study: those taking antihypertensive drugs causing PR interval prolongation (such as beta-blocking agents, verapamil, and diltiazem) and those with diabetes, structural heart disease (more than mild valvular disease, left ventricular mass index [LVMI] greater than 95 g/m2 in women and 115 g/m2 in men, grade >1 diastolic dysfunction, or LA enlargement [LA volume index (LAVI) >34 ml/m2]), sustained atrial or ventricular arrhythmia, systemic inflammatory disease, hepatic, renal or pulmonary disease, known obstructive sleep apnea, white coat hypertension or poor echogenicity. After these exclusions, 157 patients were enrolled in the study. The study protocol complied with the Helsinki Declaration, and was approved by the local ethics committee. All patients provided informed consent.
Blood pressure measurementOffice BP was measured by trained physicians in two study visits, one week apart, using a validated aneroid device with appropriate cuff sizes (Perfect Aneroid, ERKA, Bad Tölz, Germany). BP was measured after at least 5 min rest in a seated position and the measurement was repeated twice at 2-min intervals. The second and third measurements of each series were used and the mean of four measurements at two visits was determined. PP values were calculated by subtracting mean DBP from mean SBP. PP ≥60 mmHg was regarded as elevated and the patients were divided into two groups, normal and elevated PP.10
Transthoracic echocardiographyAll transthoracic echocardiography examinations were performed using a Philips iE33 xMATRIX ultrasound system with a 2.5/3.5 MHz transducer (Philips Electronics, The Netherlands), and were recorded on digital media. The recordings were later analyzed by an experienced echocardiographer who was blinded to the patients included in the study.
Left ventricular end-diastolic diameter (LVEDD) and wall thickness measurements were obtained using parasternal long-axis M-mode images. The prolate ellipse method was used to calculate LA volume.11 Apical 4-chamber and parasternal long-axis images were obtained at ventricular end-systole. In apical 4-chamber view, the distance from the mitral annular plane to the posterior wall was defined as D2, and the short-axis dimension orthogonal to D2 was defined as D1. In parasternal long-axis view, the diameter between anterior and posterior walls was recorded as D3. LA volume was calculated by the formula (D1×D2×D3)×0.523. LAVI was determined by dividing LA volume by estimated body surface area. LVMI was calculated as recommended in the current guidelines, using the formula LV mass=0.8{1.04[(LVEDD+posterior wall thickness+septal wall thickness)3-(LVEDD)3]}+0.6.12 Grade I diastolic dysfunction was defined as E/A ratio <0.8, mitral deceleration time >200 ms, septal mitral annular E wave (é) <8 cm/s and mean E/e′ ≤8, on tissue Doppler imaging.13 The PA interval was defined as the time from the onset of the P wave on the surface ECG to the onset of the A wave, which was obtained by placing the tissue Doppler sample volume over the LV lateral mitral annulus, septal mitral annulus and right ventricular tricuspid annulus. PA was calculated three times and the mean was recorded.14 Intra-right atrial conduction time was defined as septal mitral annular PA minus tricuspid annular PA; intra-left atrial conduction time was defined as lateral mitral annular PA minus septal mitral annular PA; and interatrial conduction time was defined as lateral mitral annular PA minus tricuspid annular PA.
Twelve-lead electrocardiographyA 12-lead surface ECG was recorded for all patients in the supine position using a Nihon Kohden system (Tokyo, Japan) with a paper speed of 25 mm/s and 10 mm/mV standardization. ECGs were transferred to digital media and measurements were performed manually at a magnification of 400% by two experienced cardiologists who were blinded to the study. P-wave duration was measured from the onset to the offset of the P wave in all 12 leads of the surface ECG. The difference between maximum and minimum P-wave duration was calculated and defined as P-wave dispersion (Pd). The mean Pd values obtained by two investigators were used. Intra-observer and inter-observer coefficients of variation for Pd were 3.7% and 4.8%, respectively.
Statistical analysisStatistical analyses were performed using SPSS 22.0 (IBM SPSS Inc., Chicago, IL, USA). Normally distributed continuous variables were expressed as means ± standard deviation, non-normally distributed continuous variables as medians (minimum-maximum), and categorical variables as percentages. The normality of the data was assessed with the Kolmogorov-Smirnov test. Categorical variables were compared with the chi-square test, and continuous variables were compared with either the Mann-Whitney U test or the Student's t test, as appropriate. Correlation analysis was performed using the Pearson test. Age, gender, smoking status, body mass index, serum creatinine, hemoglobin, white blood cell count, cholesterol, LVMI, LAVI, diastolic dysfunction, anti-hypertensive medication, SBP, DBP and PP were tested in univariate linear regression analysis. Multivariate regression analysis including all variables associated in univariate analysis (p<0.2) was used to identify predictors of inter- and intra-atrial EMD. A value of p<0.05 was accepted as statistically significant.
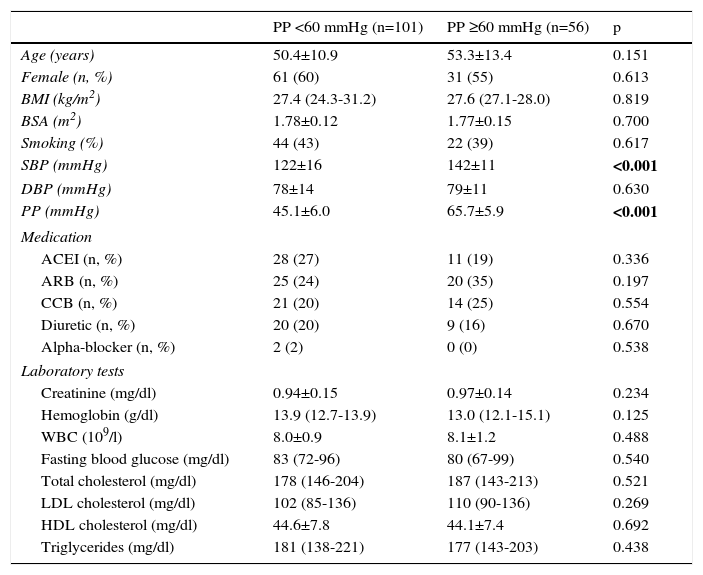
ResultsPatients’ baseline characteristics are given in Table 1. Of the 157 patients included in the study, 101 had normal PP and 56 had elevated PP. There were 61 women in the normal PP group and 31 women in the elevated PP group (60% and 55%, respectively). Apart from the difference in SBP, there was no difference between the groups with regard to baseline demographic characteristics and laboratory results. Antihypertensive treatments were similar.
Baseline characteristics of the study population.
| PP <60 mmHg (n=101) | PP ≥60 mmHg (n=56) | p | |
|---|---|---|---|
| Age (years) | 50.4±10.9 | 53.3±13.4 | 0.151 |
| Female (n, %) | 61 (60) | 31 (55) | 0.613 |
| BMI (kg/m2) | 27.4 (24.3-31.2) | 27.6 (27.1-28.0) | 0.819 |
| BSA (m2) | 1.78±0.12 | 1.77±0.15 | 0.700 |
| Smoking (%) | 44 (43) | 22 (39) | 0.617 |
| SBP (mmHg) | 122±16 | 142±11 | <0.001 |
| DBP (mmHg) | 78±14 | 79±11 | 0.630 |
| PP (mmHg) | 45.1±6.0 | 65.7±5.9 | <0.001 |
| Medication | |||
| ACEI (n, %) | 28 (27) | 11 (19) | 0.336 |
| ARB (n, %) | 25 (24) | 20 (35) | 0.197 |
| CCB (n, %) | 21 (20) | 14 (25) | 0.554 |
| Diuretic (n, %) | 20 (20) | 9 (16) | 0.670 |
| Alpha-blocker (n, %) | 2 (2) | 0 (0) | 0.538 |
| Laboratory tests | |||
| Creatinine (mg/dl) | 0.94±0.15 | 0.97±0.14 | 0.234 |
| Hemoglobin (g/dl) | 13.9 (12.7-13.9) | 13.0 (12.1-15.1) | 0.125 |
| WBC (109/l) | 8.0±0.9 | 8.1±1.2 | 0.488 |
| Fasting blood glucose (mg/dl) | 83 (72-96) | 80 (67-99) | 0.540 |
| Total cholesterol (mg/dl) | 178 (146-204) | 187 (143-213) | 0.521 |
| LDL cholesterol (mg/dl) | 102 (85-136) | 110 (90-136) | 0.269 |
| HDL cholesterol (mg/dl) | 44.6±7.8 | 44.1±7.4 | 0.692 |
| Triglycerides (mg/dl) | 181 (138-221) | 177 (143-203) | 0.438 |
ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; BMI: body mass index; BSA: body surface area; CCB: calcium channel blocker; DBP: diastolic blood pressure; HDL: high density lipoprotein; LDL: low density lipoprotein; PP: pulse pressure; SBP: systolic blood pressure; WBC: white blood cell count.
Data are presented as mean ± standard deviation or median (minimum-maximum). Bold values indicate statistical significance.
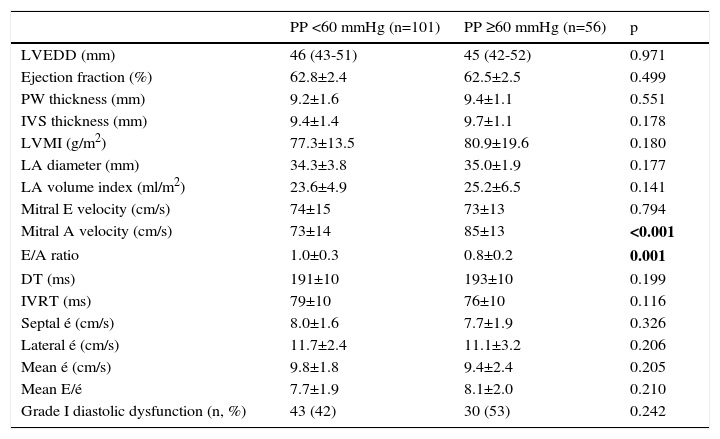
Echocardiographic data are shown in Table 2. LVMI (77.3±13.5 g/m2 vs. 80.9±19.6 g/m2, p=0.180) and LAVI (23.6±4.9 ml/m2 vs. 25.2±6.5 ml/m2, p=0.141) were higher in patients with elevated PP, but without statistical significance. The frequency of grade I diastolic dysfunction was similar in both groups (42% vs. 53%, p=0.242).
Echocardiographic parameters of the two groups.
| PP <60 mmHg (n=101) | PP ≥60 mmHg (n=56) | p | |
|---|---|---|---|
| LVEDD (mm) | 46 (43-51) | 45 (42-52) | 0.971 |
| Ejection fraction (%) | 62.8±2.4 | 62.5±2.5 | 0.499 |
| PW thickness (mm) | 9.2±1.6 | 9.4±1.1 | 0.551 |
| IVS thickness (mm) | 9.4±1.4 | 9.7±1.1 | 0.178 |
| LVMI (g/m2) | 77.3±13.5 | 80.9±19.6 | 0.180 |
| LA diameter (mm) | 34.3±3.8 | 35.0±1.9 | 0.177 |
| LA volume index (ml/m2) | 23.6±4.9 | 25.2±6.5 | 0.141 |
| Mitral E velocity (cm/s) | 74±15 | 73±13 | 0.794 |
| Mitral A velocity (cm/s) | 73±14 | 85±13 | <0.001 |
| E/A ratio | 1.0±0.3 | 0.8±0.2 | 0.001 |
| DT (ms) | 191±10 | 193±10 | 0.199 |
| IVRT (ms) | 79±10 | 76±10 | 0.116 |
| Septal é (cm/s) | 8.0±1.6 | 7.7±1.9 | 0.326 |
| Lateral é (cm/s) | 11.7±2.4 | 11.1±3.2 | 0.206 |
| Mean é (cm/s) | 9.8±1.8 | 9.4±2.4 | 0.205 |
| Mean E/é | 7.7±1.9 | 8.1±2.0 | 0.210 |
| Grade I diastolic dysfunction (n, %) | 43 (42) | 30 (53) | 0.242 |
é: mitral annular velocity; EDT: mitral E-wave deceleration time; IVRT: isovolumetric relaxation time; IVS: interventricular septum; LA: left atrium; LVEDD: left ventricular end-diastolic diameter; LVMI: left ventricular mass index; PW: posterior wall. Data are presented as mean ± standard deviation or median (minimum-maximum). Bold values indicate statistical significance.
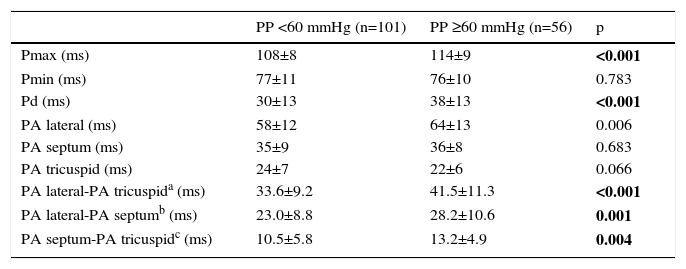
Inter-atrial EMD was 33.6±9.2 ms in patients with normal PP, and 41.5±11.3 ms in patients with elevated PP (p<0.001). Intra-left atrial EMD (23.0±8.8 ms vs. 28.2±10.6 ms, p=0.001) and intra-right atrial EMD (10.5±5.8 ms vs. 13.2±4.9 ms, p=0.004) were significantly prolonged in patients with elevated PP (Table 3). P-maximum (108±8 ms vs. 114±9 ms, p<0.001) and Pd (30±13 ms vs. 38±13 ms, p<0.001) were also prolonged in patients with elevated PP.
Comparison of electrocardiographic and tissue Doppler echocardiographic findings.
| PP <60 mmHg (n=101) | PP ≥60 mmHg (n=56) | p | |
|---|---|---|---|
| Pmax (ms) | 108±8 | 114±9 | <0.001 |
| Pmin (ms) | 77±11 | 76±10 | 0.783 |
| Pd (ms) | 30±13 | 38±13 | <0.001 |
| PA lateral (ms) | 58±12 | 64±13 | 0.006 |
| PA septum (ms) | 35±9 | 36±8 | 0.683 |
| PA tricuspid (ms) | 24±7 | 22±6 | 0.066 |
| PA lateral-PA tricuspida (ms) | 33.6±9.2 | 41.5±11.3 | <0.001 |
| PA lateral-PA septumb (ms) | 23.0±8.8 | 28.2±10.6 | 0.001 |
| PA septum-PA tricuspidc (ms) | 10.5±5.8 | 13.2±4.9 | 0.004 |
PA: time from the onset of the P-wave on surface electrocardiogram to the beginning of the A wave on tissue Doppler imaging; Pd: P-wave dispersion; Pmax: maximum P-wave duration; Pmin: minimum P-wave duration.
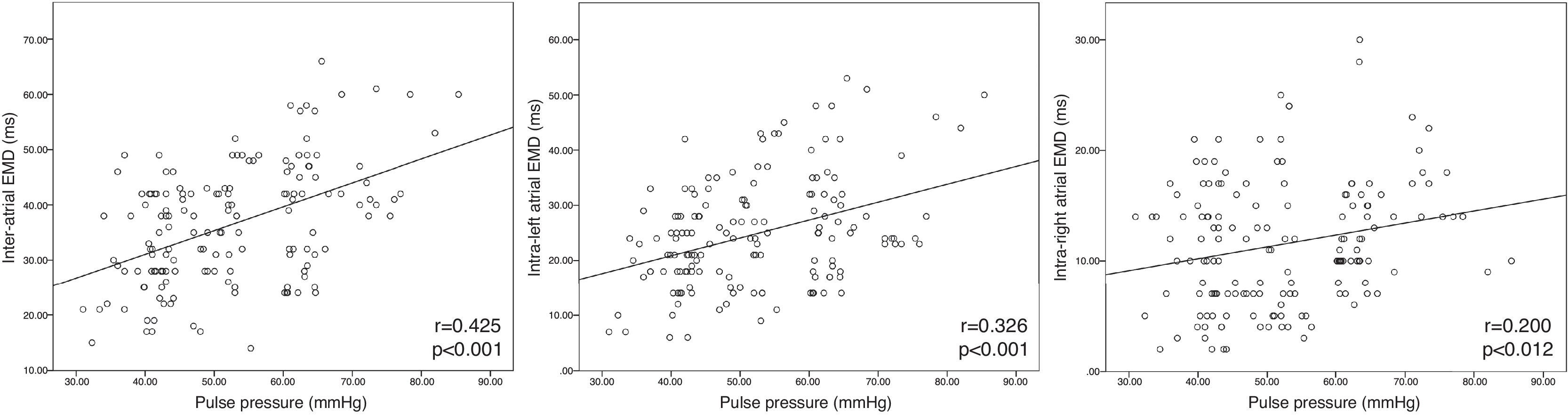
According to the correlation analysis, there was a significant positive correlation between PP and inter-atrial EMD (r=0.425, p<0.001), intra-left atrial EMD (r=0.326, p<0.001) and intra-right atrial EMD (r=0.200, p=0.012) (Figure 1). Multivariate linear regression was used to determine the effect of PP on atrial EMD. PP was independently associated with inter-atrial EMD (β=0.379, t=4.088, p<0.001) and intra-left atrial EMD (β=1.473, t=3.931, p<0.001) after controlling for potential confounders. PP lost its significance for intra-right atrial EMD in multivariate linear regression analysis (p=0.261).
DiscussionOur results show that atrial EMD and Pd were greater in patients with elevated PP and no significant cardiac structural remodeling. Moreover, PP was found to be independently associated with atrial EMD. This finding suggests that patients with elevated PP may have increased risk of AF before the development of severe structural remodeling.
Atrial EMD can easily be detected by transthoracic echocardiography, and when prolonged is an indicator of arrhythmogenic and diseased atrial tissue.7 Prolonged atrial EMD has been reported in various diseases involving electrical and structural remodeling of the atrium, including atrial septal defect,15 mitral stenosis,16 type 2 diabetes17 and some autoimmune diseases.18 It has been proposed that the conduction blockage and increased atrial conduction delay that occur in these diseases due to atrial fibrosis and remodeling could be a trigger and driving force for AF through the development of reentry loops.
As is known, increased BP is an important risk factor for AF.19 Office PP has been found to be independently associated with cardiovascular morbidity and mortality and correlates strongly with ambulatory blood pressure monitoring (ABPM).20 Previous studies have reported that office SBP and PP are independent risk factors for AF, while mean BP and DBP are not.4 Okin et al. noted the importance of SBP in new-onset AF.21 However, as a pulsatile component of BP, PP elevation resulting from either increased SBP or decreased DBP is a more powerful predictor of AF development than other BP parameters.22 Although PP is regarded as an indirect indicator of aortic stiffness, according to Roetker et al. the association between AF and PP cannot be explained by aortic stiffness, and they proposed PP as an independent predictor of AF.6 It is not clear how increased PP produces an AF substrate in the atrium. It is thought that acute elevations in PP together with chronic remodeling processes occurring in the left ventricle and left atrium may play a role in new-onset AF by increasing LA wall tension.23 Furthermore, AF development may also be triggered by PP elevation leading to neurohumoral activation24 and cardiovascular inflammation.25 Electrophysiologically, hypertension leads to global atrial conduction disturbances and facilitates AF inducibility.26 In our study, higher intra-left atrial and inter-atrial EMD values and prolonged Pd in patients with elevated PP were indicators of slow atrial conduction. These findings suggest that electrophysiological remodeling in hypertensive patients is more prominent in those with elevated PP. Although an independent predictor of AF on its own, elevated PP is often found together with additional conditions that are known to be closely associated with AF. A higher prevalence of complications such as left ventricular hypertrophy, LA enlargement and diastolic dysfunction in patients with elevated PP will tend to favor the development of an AF substrate.5,6,27 In our study population, none of the complications of hypertension in the left heart were observed more frequently in patients with elevated PP. Patients with moderate and severe diastolic dysfunction were excluded from our study, based on the assumption that the effect of PP on atrial EMD may be obscured in these patients.28 The mechanism by which PP was a predictor of atrial EMD independently of LAVI, LVMI, SBP and mild diastolic dysfunction on multivariate analysis is not clear; however this finding may be a reflection of increased LA wall tension. Some studies have found that increased atrial natriuretic peptide levels resulting from increased LA wall tension are associated with AF.29 Furthermore, acute elevation in PP may cause an abrupt increase in LA pressure, and can predict recurrence of AF.30 The pathogenesis of the independent arrhythmogenic effect of PP is not fully understood and it is not clear whether setting control of PP as a clinical target would help to decrease the incidence of newly diagnosed AF. Antihypertensive treatments decrease AF frequency by decreasing atrial dilatation and fibrosis and the atrial refractory period.31 Moreover, regression in LA size achieved by antihypertensive treatment decreases new-onset AF attacks.32 Control of PP optimizes control of left intra-atrial pressure in hypertensive patients, and therefore may reduce the frequency of new-onset AF by decreasing tension-related waves originating from the superior pulmonary veins.30,33 In our study, there was no difference between the two groups with regard to various antihypertensive drugs.
Study limitationsOne limitation of our study is the relatively small number of patients. Although the prolate ellipse method used to calculate LAVI has been reported to give relatively low LA volume values, especially in patients with increased anteroposterior diameter, there was no apparent enlargement in this diameter in our patient group.11 Another limitation was the fact that LA wall tension and pressure were not assessed, which could have been relevant to the etiopathogenesis of AF. Assessment of BP by ABPM would also have given additional information and might have helped to diagnose white coat hypertension and masked hypertension. Types of drugs and their dosages might have influenced the results, but this would have been difficult to control for given the large number of antihypertensive agents used and their different dosages.
ConclusionIn conclusion, our study showed that elevated PP is associated with higher atrial EMD and Pd in the early stages of hypertension before the development of significant structural remodeling. Elevated PP is a contributor to increased atrial EMD independently of SBP, LAVI, LVMI and mild diastolic dysfunction. These findings may help to understand the greater tendency of patients with elevated PP to develop AF. Further studies are needed to understand atrial electrical remodeling in patients with elevated PP.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflicts of interest to declare.