The aim of this study was to analyze the incidence of drug-eluting stent thrombosis (sirolimus or everolimus) in patients with chronic total coronary occlusions (CTO) and to determine its clinical implications and related factors.
MethodsData from the 12-month follow-up of the 207 patients included in the CIBELES trial with CTO were analyzed.
ResultsStent thrombosis occurred in three patients, two definite and one probable (overall thrombosis rate: 1.4%). However, there were no cases of death or Q-wave myocardial infarction. In univariate analysis, patients with a higher incidence of stent thrombosis were those in whom the target vessel was the left anterior descending, who had single-vessel disease, were assigned to treatment with sirolimus-eluting stents, and those with smaller minimum luminal diameter immediately after the procedure. In multivariate analysis, the only independent predictor of stent thrombosis was minimal luminal diameter immediately after the procedure.
ConclusionsThe rate of drug-eluting stent thrombosis in patients with CTO is relatively low (1.4%). The only independent predictor of stent thrombosis in this context was minimal luminal diameter after the procedure and the clinical presentation was in all cases relatively benign.
o objetivo do nosso estudo foi analisar a incidência de trombose de stent com eluição de fármaco (sirolimus ou everolimus) em pacientes com oclusões coronárias crónicas e identificar as suas implicações clínicas e fatores relacionados.
Métodos12 meses de acompanhamento dos 207 pacientes incluídos no ensaio Cibeles com oclusão total coronária crónica.
Resultadosa trombose de stent ocorreu em três doentes: duas definitivas e uma provável (taxa global de trombose 1,4%). No entanto, não surgiu nenhum caso de morte ou enfarte do miocárdio com onda Q. Na análise univariada, os doentes com maior incidência de trombose de stent foram aqueles em que o vaso-alvo foi a descendente anterior, os que tinham a doença de um vaso, os que foram considerados para tratamento com stent com sirolimus, e aqueles com diâmetro luminal mínimo menor imediatamente após o procedimento. Na análise multivariada, o único preditor independente de trombose do stent foi o diâmetro luminal mínimo imediatamente após o procedimento.
Conclusõesa taxa de trombose de stent farmacológico em pacientes com oclusão coronária crónica foi relativamente baixa (1,4%).
O único preditor independente de trombose de stent neste contexto foi o diâmetro luminal mínimo após o procedimento, tendo a apresentação clínica em todos os casos sido relativamente benigna.
chronic total occlusion
stent thrombosis
everolimus-eluting stent
sirolimus-eluting stent
drug-eluting stent
bare-metal stent
Chronic total coronary occlusions (CTO) constitute one of the most complex and challenging therapeutic scenarios for an interventional cardiologist, due not only to the technical complexity of the lesion and the need for specific operator skills, but also to the many complications that can occur during and after the procedure.1–4 Stent thrombosis (ST) represents the most important clinical complication due to the high rate of death and/or myocardial infarction.5 Its pathophysiology includes various factors related to the device itself, the procedure and the patient.6
It has been traditionally considered that one the factors associated with increased risk of ST is, as suggested by some studies,7,8 the implantation of a drug-eluting stent (DES) in the context of a CTO. The aim of this study is to assess the incidence, clinical significance and timing of DES thrombosis in the treatment of CTO, given the lack of clear knowledge concerning the safety of these devices in this context. For this purpose, data on ST from the CIBELES trial9 were studied.
MethodsPatientsThe CIBELES clinical trial (ClinicalTrials.gov number NCT00793221) included 207 patients with CTO of more than two weeks duration, after successful balloon angioplasty. These patients were randomized to receive an everolimus-eluting stent (EES) (Xience V, Abbott Vascular) or a sirolimus-eluting stent (SES) (Cypher, Cordis). The primary outcome was in-stent late loss. Thirteen centers in Spain and Portugal were involved in this trial, which was sponsored by the Spanish Society of Cardiology. Abbott Vascular supported the study by an unconditional grant.
The antithrombotic therapy, approach (radial or femoral) and material used during the procedure were chosen according to operator preferences or the established protocols of each center. Angiographic follow-up was performed at nine months after revascularization. In the event of significant restenosis, repeat revascularization was performed, if clinically indicated (angina, silent ischemia or presence of myocardial viability) and technically feasible. Quantitative coronary analysis was performed at an independent laboratory using MEDIS software (Leiden, The Netherlands). The study was monitored by Chiltern International, and featured an independent clinical events committee. The clinical follow-up was performed over a period of 12 months after revascularization.
This substudy assesses the incidence, timing, clinical presentation and independent predictors of ST in patients included in the CIBELES trial.
DefinitionsThe definition of ST used was that of the Academic Research Consortium,10 as follows:
- (1)
definite thrombosis: documented angiographically or by autopsy;
- (2)
probable thrombosis: an acute coronary syndrome in the territory of the treated artery, or either sudden cardiac or unexplained death in the 30 days after stent implantation;
- (3)
possible thrombosis: sudden cardiac or unexplained death beyond 30 days after stent implantation.
In view of these criteria, of special interest for the study were the rates of definite or probable thrombosis. From a chronological point of view, thrombosis was considered as:
- (1)
acute: within 24 hours after stent implantation;
- (2)
sub-acute: between the first 24 hours and the first 30 days after stent implantation;
- (3)
late: beyond the first 30 days after stent implantation.
The collected data were analyzed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Qualitative variables were expressed as percentages, and quantitative variables as mean ± standard deviation. The chi-square test and Fisher's exact test were used to compare qualitative variables. Multivariate analysis was performed using logistic regression analysis. Comparison between quantitative variables was performed using the Student's t test. A value of p<0.05 was considered statistically significant.
ResultsIncidence and clinical presentation of stent thrombosisThe clinical and angiographic characteristics of the study population are summarized in Tables 1 and 2. During follow-up, ST was diagnosed in three patients, two definite (1 and 117 days after the procedure) and one probable (nine days after the procedure). The characteristics of these patients are summarized in Table 3.
Clinical characteristics of the study population.
| No thrombosis | Thrombosis | p | |
|---|---|---|---|
| Age (years) | 67±6 | 64±10 | 0.493 |
| Female (%) | 0 | 17.3 | 1.000 |
| Diabetes (%) | 36.8 | 0.0 | 0.555 |
| Hypertension (%) | 67.6 | 100.0 | 0.553 |
| Hypercholesterolemia (%) | 66.7 | 71.6 | 1.000 |
| Smoking (%) | 100.0 | 54.9 | 0.256 |
| PAD (%) | 0.0 | 11.8 | 1.000 |
| LVEF (%) | 48±8 | 53±13 | 0.568 |
| Creatinine (mg/dl) | 0.8±0.1 | 1.0±0.4 | 0.499 |
| Blood glucose (mg/dl) | 93±22 | 120±50 | 0.499 |
| Previous PCI (%) | 67.7 | 33.3 | 0.265 |
| Previous surgery (%) | 0.0 | 4.4 | 1.000 |
| Previous MI (%) | 33.3 | 37.7 | 1.000 |
LVEF: left ventricular ejection fraction; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention.
Angiographic characteristics of the study population.
| Thrombosis | No thrombosis | p | |
|---|---|---|---|
| Number of affected vessels | 1±0 | 1.8±0.8 | 0.080 |
| Number of stents | 2.7±0.6 | 2.0±1.0 | 0.254 |
| Maximum stent diameter (mm) | 2.8±0.3 | 2.9±0.4 | 0.438 |
| Stent length (mm) | 59.7±19.0 | 48.7±24.2 | 0.422 |
| Reference diameter (mm) | 2.7±0.8 | 3.0±0.6 | 0.694 |
| Minimal luminal diameter (mm) | 1.3±0.6 | 2.2±0.5 | 0.002 |
| Post-procedural stenosis (%) | 42.8±13.7 | 20.4±9.7 | <0.001 |
| Post-procedural TIMI flow | 2.7±0.6 | 2.9±0.4 | 0.288 |
| Balloon over-dilation | 0.0±0.0 | 0.3±0.4 | 0.313 |
| Multivessel disease (%) | 0.0 | 56.9 | 0.049 |
| Location anterior descending (%) | 100.0 | 40.2 | 0.037 |
| Bifurcation (%) | 0.0 | 25.0 | 1.000 |
| Radial approach (%) | 0.0 | 23.5 | 1.000 |
| Retrograde access (%) | 0.0 | 4.9 | 1.000 |
| Anticoagulant used (%) | |||
| Sodium heparin | 66.7 | 82.8 | 0.438 |
| Bivalirudin | 33.3 | 11.8 | 0.322 |
| Enoxaparin | 0.0 | 5.4 | 1.000 |
| Time of occlusion >3 months (%) | 66.7 | 79.9 | 0.495 |
Characteristics and clinical presentation of patients with stent thrombosis.
| Type | Stent | Time | Presentation | |
|---|---|---|---|---|
| 1 | Definite | SES | 24 hours | Angina |
| 2 | Probable | SES | 9 days | NSTEMI |
| 3 | Definite | SES | 117 days | NSTEMI |
| 4 | Possible | EES | 8 month | Sudden death |
EES: everolimus-eluting stent; NSTEMI: non-ST-elevation myocardial infarction; SES: sirolimus-eluting stent.
The first patient was randomized to the SES group and received three stents. However, a paclitaxel-eluting stent was also implanted distally to the treated lesion. Within 24 hours of the procedure the patient presented acute ST of the paclitaxel device. Although there was no evidence of thrombosis of the SES, this event was classified as definite ST as the design of the analysis was by intention to treat.
The second patient experienced a non-ST-elevation acute coronary syndrome nine days after the initial intervention. This event was initially treated conservatively. At the scheduled angiographic follow-up, coronary angiography demonstrated total occlusion of the target vessel. ST was considered the most likely cause of the event.
A third patient had a definite ST, 117 days after treatment. This patient was admitted with a non-ST-elevation acute coronary syndrome; angiography showed a thrombotic occlusion of the treated target vessel and PCI was subsequently repeated.
The overall rate of definite or probable thrombosis was therefore 1.4% (0.9% early, 0.5% late). In no case did ST lead to death or Q-wave myocardial infarction. The clinical presentation was non-Q-wave myocardial infarction in two cases and unstable angina in one. There were also no documented cases of probable or definite ST in patients assigned to the EES group.
A fourth patient had sudden death of unknown cause eight months after the coronary intervention. ST was considered in this case as possible. Thus, the overall incidence of ST (definite, probable or possible) was 1.8%.
Factors related to stent thrombosisThe univariate analysis (Table 2) revealed that patients with a higher incidence of ST were those who had single-vessel disease (3.3% vs. 0.0%, p=0.049), in whom the lesion was in the anterior descending artery (3.5% vs. 0.0%, p=0.037), and who showed a lower minimum luminal diameter (1.3±0.6 mm vs. 2.6±0.5 mm, p<0.001) immediately after the procedure. Moreover, the ST rate was 3.0% in the SES group compared to 0.0% in the EES group (p=0.074) (Figure 1). Multivariate analysis showed that the only independent predictor of ST in this context was minimal lumen diameter immediately after the procedure (p=0.012).
DiscussionST is an uncommon event, with a variable clinical presentation but generally unfavorable course, resulting in high rates of myocardial infarction (50–70%) or death (20–40%). The data in the literature on its incidence are inconsistent, but it is estimated at between 0.5% and 2.5%. These figures are slightly lower and with earlier clinical presentation for bare-metal stents (BMS).9
The essential pathophysiology is closely related to various parameters and chronological stages: early events often depend on variables associated with the procedure itself, including the presence of residual thrombus, dissection in the proximity of the target lesion, stasis, stent under-expansion, stent length, or a combination of the above. Late events are most frequently associated with delayed scarring or incomplete and/or inadequate neointimal covering. Additionally, underlying vascular inflammation and the length of the implanted devices have also been shown to be important, with patients who had received longer stents or experienced an allergic reaction to the polymer most often suffering ST.11–13
Traditionally, percutaneous revascularization of a CTO is considered to be at high risk for ST. This is due to the complexity of the procedure and the intricate histopathology of these lesions, which contain varying amounts of collagen, lipid, calcifications and intraluminal microchannels. All this leads to increased vulnerability and susceptibility to vascular damage.14,15 Numerous clinical studies have demonstrated the superiority of DES (mainly SES) compared with BMS in the treatment of CTO,16–21 with a lower restenosis rate and fewer major adverse cardiac events. There are no data in the literature that conclusively demonstrate increased rates of ST in this context. However, ST is slightly more frequent in patients treated with first-generation DES.
The new generations of DES have been developed to improve safety and biocompatibility, optimizing the three main components: the platform, the polymer and the drug. They have been shown to be more effective than the first generation, mainly due to a reduction in the rate of medium-term target vessel revascularization. They have also shown a better safety profile, with lower rates of ST. These findings have been supported by the results of numerous clinical trials published in recent years (SPIRIT II,22 SPIRIT III,23 SPIRIT IV,24 COMPARE I25), which compared the two generations of DES.
So the results of the CIBELES trial, which demonstrates the non-inferiority of EES versus SES, should not surprise us. They corroborate the previously published data and establish the safety of EES in the context of CTO. Although it is not possible to draw definite conclusions concerning differences in ST between the two treatment groups, there were no cases of definite or probable thrombosis in the EES arm. Other second-generation stents, such as zotarolimus-eluting stents, have been proven to be at least as effective as SES in the treatment of CTO, offering similar results in terms of numbers of ST.26
As noted above, ST usually presents as an acute event with a variable spectrum, but usually with high rates of morbidity and mortality. However, in our study the clinical course was relatively benign, presenting in all cases as an episode of unstable angina or a nonfatal non-ST-elevation myocardial infarction. The particular angiographic scenario probably contributed significantly to this clinical course, as pre-existing collateral circulation may have played a protective role in the event of an ischemic event caused by ST.
The data obtained suggest that the only independent predictor of ST is minimal luminal diameter immediately after the procedure. This suggests that stents implanted in small vessels or malpositioned or under-expanded (due to, for example, the existence of underlying calcium) are at higher risk of thrombosis. Recently published data emphasize the low use of non-compliant balloons in the treatment of CTO. In the CIBELES trial, post-dilatation was only performed in 25% of patients, a figure that is surprising given the expected proportion of malpositioning or under-expansion of the implanted stents according to the type and length of the treated lesions. Invasive imaging techniques such as IVUS guided the procedure and its outcome in only 6% of cases.27
It is known that the same precipitating factors are predictors of ST after revascularization with DES in other coronary lesions.28,29 Analyzing the timing of the event rate, there is no evidence of a clear grouping, with one acute, one subacute, and one late ST in our study. The two early events were presumably related to the procedure itself and associated local complications. Stenting often leads to areas of dissection along the artery, and together with the presence of organized thrombotic material, areas of calcification and suboptimal end TIMI flow, would provide the pathophysiological basis for increased incidence of ST. However, and despite the complexity of the procedure, the frequency of acute and subacute ST is very low, representing only 0.97% of all our cases.
Furthermore, thrombosis associated with DES occurs later than with BMS.28 The main cause of this phenomenon is delayed endothelialization of the device, caused by the antiproliferative action of the drug, as indicated by various angioscopy, optical coherence tomography and autopsy studies, although no definite conclusions can be drawn.30,31 We found only one case of late ST, but the follow-up in our study was only 12 months after revascularization. Hence, possible very late thrombosis events were not recorded.
In light of the above, we consider that this substudy of the CIBELES trial shows that ST in the context of CTO treated with DES is an uncommon phenomenon, similar to that of other coronary lesions. The previously known predisposing factors account for the majority of the risk, although the importance of certain local factors, such as the frequent presence of underlying calcium or areas of arterial dissection, should be borne in mind. Our data are similar to those published by other groups, in which the efficacy of EES32 and other second-generation stents33,34 was assessed, suggesting the existence of a class effect.
Moreover, second-generation DES have tended to diminish ST and are probably better in this regard than the first generation. The clinical presentation is in this context milder than in other circumstances, as the abundant collateral circulation acts as insurance and protects possible at-risk myocardial territory. Therefore, and given the available scientific evidence, we believe that DES stenting in CTO – and even more with second-generation stents – does not lead to added risk of ST, and presents a clear benefit in restenosis rates.21 Invasive imaging techniques such as IVUS or optical coherence tomography, and the use of non-compliant balloons after stent implantation, are useful tools to predict and correct malpositioning or under-expansion, thereby alleviating some of the main triggers of thrombosis.
Study limitationsOur study has some limitations. First, the CIBELES trial was designed to compare stent restenosis with EES and SES, and not to study ST. However, it is not really feasible to perform a specific clinical trial to assess ST in the context of CTO. Secondly, CTO were defined as those with estimated occlusion time >2 weeks (and not >3 months as recommended in recent consensus documents). The data are thus comparable to those of other trials of DES in CTO, such as PRISON II.21 In any case, in our study the estimated duration of occlusion was not related to the rate of ST. Thirdly, since the choice of antiplatelet therapy was at the discretion of the operator, there was no uniformity concerning the dose and type of drug administered. Finally, the clinical monitoring of patients was conducted over a 12-month period, so events after this period were not recorded. Consequently, despite its low incidence, possible very late ST is not included in this study.
ConclusionsThe rate of thrombosis of DES in patients with CTO is low (1.4%), especially in those treated with EES compared to SES. In this context, the only independent predictor is minimal luminal diameter after the procedure. The clinical presentation in all cases was relatively benign (unstable angina or nonfatal non-ST-elevation myocardial infarction). The use of DES in the treatment of CTO is a safe option and should not be considered to increase risk of thrombosis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThis study was supported by the Spanish Society of Cardiology, with an unrestricted grant by Abbott Vascular.








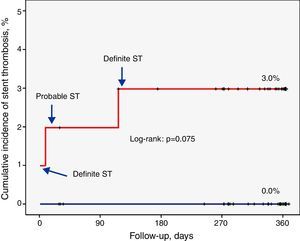 EES: everolimus-eluting stent;
EES: everolimus-eluting stent; 

