Antiarrhythmic drugs are often the last resort for recurrent ventricular tachycardia refractory to catheter ablation in implantable cardioverter-defibrillator carriers. Amiodarone, alone or combined with mexiletine, is usually but not always highly effective, and its use is usually limited by systemic adverse effects. We present the case of a 62 years old man with recurrent ICD shocks due to a VT refractory to an endo-epicardial hybrid ablation. Starting of dronedarone plus mexiletine combination showed an excellent result.
As drogas antiarrítmicas são frequentemente o último recurso para taquicardia ventricular (TV) recorrente refratária à ablação por cateter em portadores de cardioversor-desfibrilador implantável (CDI). A amiodarona, isolada ou combinada com mexiletina, é altamente, mas nem sempre, eficaz e o seu uso é geralmente limitado por efeitos adversos sistémicos. Apresentamos o caso de um homem de 62 anos com choques recorrentes do CDI devido a uma TV refratária a uma ablação híbrida endoepicárdica. O início da combinação de dronedarona e mexiletina mostrou um excelente resultado.
Management of recurrent ventricular tachycardia (VT) in structural heart disease can be complex. A stepwise approach with antiarrhythmic drugs (AADs) as well as catheter ablation is frequently required. Amiodarone is the most effective AAD but its use is limited by its systemic adverse events. Dronedarone is an analog of amiodarone with fewer side effects and with proven efficacy in atrial fibrillation,1 but it has not been approved for VT treatment. Furthermore, no evidence has been reported of dronedarone added to mexiletine. We describe our experience with this combination.
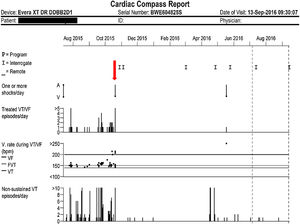
Case reportA 62-year-old man with a mechanical aortic valve and an implantable cardioverter-defibrillator (ICD) implanted 13 years previously was referred to our arrhythmia unit due to episodes of refractory VT. The patient did not have coronary artery disease and left ventricular ejection fraction was 48%. Recurrent device therapies due to VT episodes were refractory to sotalol and to amiodarone plus beta-blockers combined with mexiletine, so in 2014 catheter ablation of the endocardial substrate was planned. Although no inducibility was achieved, one month later the patient experienced VT relapse that required intravenous procainamide. At discharge, amiodarone plus mexiletine was resumed, but episodes continued and amiodarone had to be withdrawn because of hyperthyroidism. After thyroid function was controlled, the patient was referred to a specialist VT ablation unit. Hybrid endocardial-epicardial ablation was performed with median sternotomy surgical access. Epicardial submitral and septal intramyocardial substrates were identified. Despite ablation, clinical VT related to a septal subprosthetic substrate remained inducible. Briefly, after the procedure, on beta-blockers plus mexiletine, VT episodes recurred and were aborted by ICD antitachycardia pacing and shocks. Given the setting of refractory VT, the patient was referred for heart transplantation. While he was on the waiting list, 400 mg twice daily of dronedarone was initiated, added to mexiletine 100 mg twice daily and bisoprolol 5 mg twice daily. Eleven months after dronedarone was started, the VT burden was dramatically reduced, only one ICD therapy was delivered, and the drug combination was well tolerated without adverse effects. The lower cutoff for the VT detection zone had been programmed for 36 intervals above 140 bpm (Figure 1).
Graphical view of treated ventricular tachycardia/ventricular fibrillation episodes/day (top) and non-sustained ventricular tachycardia episodes/day (bottom). The y-axis represents the number of episodes and the x-axis represents time in months. The vertical downward red arrow indicates the visit when dronedarone was started, in mid-October 2015.
Dronedarone is a class III antiarrhythmic drug, a non-iodinated analog of amiodarone, with proven efficacy in atrial fibrillation.1 Amiodarone is to date the most effective drug for the management of malignant VT in ICD carriers, with a good cardiac safety profile. Its combination with mexiletine has been shown to increase efficacy, reducing the frequency of VT events in ICD carriers.2 However, its long-term use is limited by non-cardiac adverse effects that necessitate withdrawal. Dronedarone has a better safety profile,1 but its efficacy has not been proven in humans for treating VT. Animal trials have shown even higher efficacy in VT than that of amiodarone.3 In humans, only isolated case reports suggest that it is likely to be effective in this setting,4 but no experience has been reported in combination with mexiletine. Our case suggests very high mid-term efficacy of this combination in a patient refractory to all possible therapies excepting heart transplantation, the only option for him before administration of dronedarone plus mexiletine. These drugs dramatically reduced his VT burden, and we were able to remove him from the transplantation waiting list. The sparse but striking evidence of their efficacy in our patient suggests the need for human trials to confirm our finding and to lead to their on-label use.
Conflicts of interestThe authors have no conflicts of interest to declare.