Congenital coronary fistulas are rare conditions, frequently diagnosed as an incidental finding when a patient is referred for cardiac surgery for another reason. Treatment can be conservative, surgical or more recently through transcatheter closure, depending on local experience and the morphology of the fistula.
The authors present the case of a pediatric patient with a large coronary artery fistula from the aorta to the right atrium. Transcatheter closure with a 16 mm Amplatzer® vascular plug II and a 6 mm Amplatzer® duct occluder was performed, with complete occlusion.
As fístulas coronárias congénitas constituem uma patologia rara, frequentemente de diagnóstico acidental, por exemplo durante uma cirurgia cardíaca por outro motivo. A terapêutica pode ser conservadora, cirúrgica ou por intervenção percutânea, dependendo da experiência local ou da morfologia da fístula.
É apresentado um caso de um paciente pediátrico com um diagnóstico de fístula coronária gigante da aorta com drenagem na aurícula direita. Foi efetuado encerramento percutâneo com um dispositivo Amplatzer® Vascular Plug II de 16 mm e um dispositivo Amplatzer Duct Occluder de 6 mm, com oclusão total da fístula.
Coronary fistulas are rare congenital heart malformations that result in a connection between one or more of the coronary arteries and a cardiac chamber or great vessel, bypassing the myocardial capillary bed.1,2 In about half of cases the fistula originates from the right coronary artery, in one third from the left anterior descending artery and in about one-fifth from the circumflex artery.1 The majority drain to the right side of the heart. Drainage, which can occur through one or multiple orifices, is more frequently into the right ventricle, followed by drainage into the right atrium.1 Either of these possibilities will cause volume overload in right-sided structures and increased pulmonary vascular flow, similar to a left-to-right shunt from an atrial septal defect, ventricular septal defect or patent ductus arteriosus. Coronary fistulas are usually asymptomatic until the second decade of life. Occasionally a continuous murmur can be heard or cardiomegaly may be accidentally detected on a chest X-ray. Myocardial ischemia is also a possibility, usually in adult patients, due to coronary artery steal.1 Other complications may include thrombosis, embolism, cardiac failure, atrial fibrillation, aneurysmal dilatation and rupture, endocarditis, endarteritis or arrhythmias.1,4 Although the electrocardiogram (ECG) and chest X-ray can be helpful and cardiac magnetic resonance imaging is of increasing importance, the main diagnostic technique remains cardiac catheterization and angiography, which provides information regarding the hemodynamic significance, location and size of the fistula.1 Interventional catheterization techniques and surgery are both useful in closure of these vascular abnormalities.3
Case reportA ten-year-old girl with no previous relevant medical history was referred for a pediatric cardiology assessment due to increased cardiothoracic ratio of 0.6. On physical examination she presented a grade 2/6 continuous murmur at the upper and middle sternal border and normal arterial pulses. The ECG was normal and the echocardiogram revealed a tubular structure (fistula) from the aorta to the right atrium. The right-sided chambers were slightly enlarged but ventricular contractility was normal.
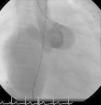
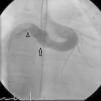
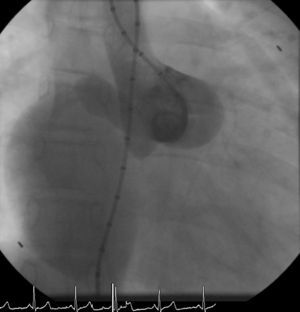
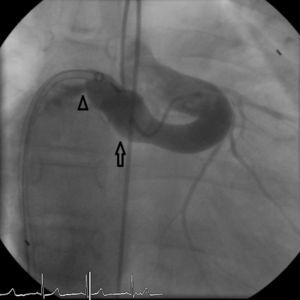
The patient was referred for a diagnostic cardiac catheterization. Angiography confirmed a coronary fistula measuring 13 mm in diameter at the aortic end and draining into the right atrium (RA) through at least two small openings (Figure 1). The left anterior descending (LAD) and circumflex (Cx) arteries originated in the proximal extremity of the fistula through two separate orifices. Catheterization showed normal right and left heart pressures, oxygen saturation step-up in the right atrium and Qp:Qs of 1.7. Informed consent was obtained and percutaneous closure of the fistula was attempted using a 16 mm Amplatzer® vascular plug II (AGA Medical). Through a femoral approach, a 7 Fr venous and a 6 Fr arterial sheath were used. A 6 Fr Concierge Amplatz Left 2 guiding catheter was positioned in the aorta at the fistula entrance and a 0.035 in Terumo® hydrophilic guide wire was advanced along the fistula, until the right atrium was reached. The wire was then snared and an arteriovenous (AV) loop was created. A 7 Fr Amplatzer Delivery System was used to deploy the device through the atrial end (Figure 2). A significant residual shunt was observed after deployment of the device. Cardiac enzymes were within normal range and the ECG showed no abnormalities. After this procedure anticoagulation was prescribed for six months and aspirin maintained thereafter.
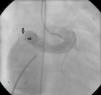
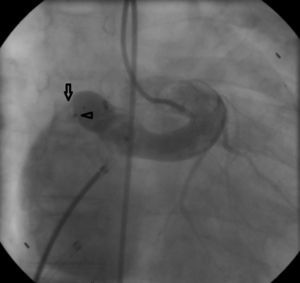
Regular follow-up was performed and transthoracic echocardiography detected a residual shunt at the distal extremity of the fistula. One year after the first intervention, angiography was repeated and two small orifices were clearly seen in the extremity where the fistula drained into the right atrium. Percutaneous closure of these orifices was attempted. A 6 Fr venous sheath and a 6 Fr arterial sheath were used through a femoral approach. Two guide wires were advanced from the aortic end to the atrial end, one through each orifice, and two arteriovenous loops were created. Two 6 Fr Concierge Amplatz Left 2 guiding catheters were introduced in the distal extremity of the fistula. An 8/6 mm Amplatzer® duct occluder (AGA Medical) was deployed through the atrial end to close the lower orifice (Figure 3). The upper orifice was left open, due to dislocation and instability in the position of the guiding catheter in the distal part of the fistula, and the long duration of the procedure. Control angiography showed a residual shunt through the upper orifice.
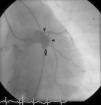
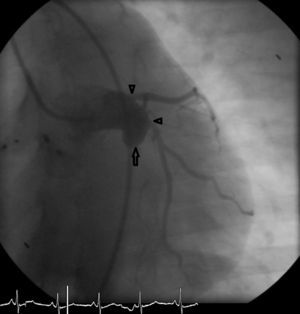
Nine months after the second intervention, coronary angiography was repeated. No fistulous flow was detected, the fistula being occluded 2 cm before the emergence of the Cx artery (Figure 4). LAD and Cx artery flows were normal. Following the second procedure the patient remains asymptomatic and well. Antiplatelet therapy with aspirin was continued.
DiscussionComplications related to coronary artery fistulas have been used as an argument to justify intervention, either surgical or percutaneous. Indications for closure include the presence of a large left-to-right shunt, left ventricular volume overload, left ventricular dysfunction, myocardial ischemia, congestive cardiac failure, and prevention of endocarditis.1,4 Several devices have been used for percutaneous closure of these vascular malformations, including the Amplatzer® vascular plug,5 Amplatzer® duct occluder,6 Amplatzer® muscular VSD device and coils,4,5,7 but controversy remains as to the best approach. The choice of equipment, device and technique clearly depend on the morphology of the lesion, the experience of the attending cardiologist, and the patient's age and size. This case report highlights the difficulty the interventional cardiologist faces when approaching these malformations, since two devices had to be implanted before occlusion of the coronary fistula could be achieved. Although a small orifice was left open after the second percutaneous intervention due to technical difficulties in the placement of the catheter, a successful result was achieved. A possible explanation for this could be the sudden decrease of flow after occlusion of one of the orifices at the atrial extremity of the fistula.
Possible complications of closure of a coronary artery fistula include embolization of the device, myocardial ischemia and ECG abnormalities including T-wave changes and bundle branch block.1,2,7
Conflicts of interestThe authors have no conflicts of interest to declare.