Left ventricular noncompaction is an unusual but increasingly recognized cardiomyopathy, the etiology of which is still not definitely established. Clinical presentation includes a wide spectrum of scenarios, including heart failure, thromboembolism and malignant arrhythmias, with half of deaths occurring suddenly. Early detection of LVNC is therefore essential to prevent sudden cardiac death. To our knowledge, this is the first report of the presence of cardiac sympathetic nervous dysfunction, assessed by 123iodine-metaiodobenzylguanidine myocardial scintigraphy, in a patient with LVNC, preserved left ventricular systolic function and exercise-induced nonsustained ventricular tachycardia. This finding may be related to the increased arrhythmic risk observed in this cardiomyopathy, giving a new insight into the pathophysiology of LVNC.
O miocárdio não-compactado (MNC) é uma miocardiopatia que, embora rara, tem sido gradualmente mais descrita. O espectro de apresentação clínica é variável, incluindo insuficiência cardíaca, fenómenos tromboembólicos e arritmias, sendo que metade das mortes ocorre subitamente (MSC). Por conseguinte, o diagnóstico precoce e correto do MNC é fundamental para a prevenção da MSC. O presente artigo ilustra pela primeira vez a presença de disfunção neuronal simpática, avaliada por cintigrafia cardíaca com 123I-metaiodobenzilguinadina, num doente com MNC, função sistólica ventricular esquerda preservada e taquicardia não sustentada induzida pelo esforço. A presença de alterações na actividade adrenérgica cardíaca pode estar relacionada com o maior risco arrítmico característico desta miocardiopatia, podendo contribuir para o melhor esclarecimento da sua patofisiologia.
Left ventricular noncompaction (LVNC) is a genetic primary cardiomyopathy characterized by a distinctive spongy appearance of the myocardium due to prominent trabeculations and deep intertrabecular recesses. These features are usually more prominent in the apex and/or mid segments of both left ventricular (LV) inferior and lateral walls. Although the cause of LVNC is not completely understood, the disease appears to result from intrauterine arrest of compaction of the loose myocardial meshwork. Heart failure (HF), arrhythmias and thromboembolic events are frequent clinical manifestations and also major morbidity factors during long-term follow-up. In this case report, we describe for the first time the evidence of abnormal cardiac sympathetic nervous function through 123iodine-metaiodobenzylguanidine myocardial scintigraphy (123I-MIBG) in a patient with preserved LV systolic function and exercise-induced nonsustained ventricular tachycardia.
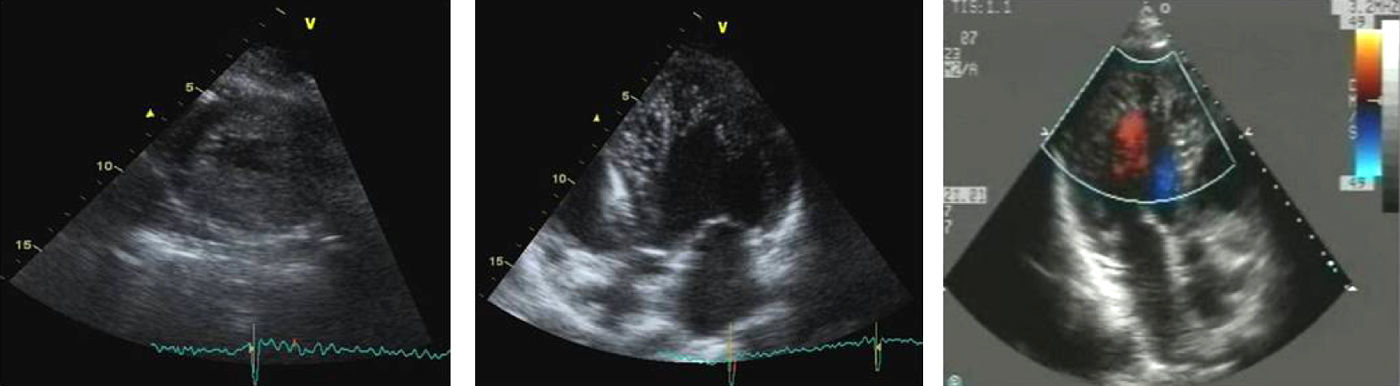
Case reportAn asymptomatic 32-year-old man with a family history of sudden cardiac death (SCD) (mother and maternal uncle died at ages 33 and 30, respectively) with no relevant findings on physical examination, underwent a routine transthoracic echocardiogram that revealed a non-enlarged LV (end-diastolic diameter of 56mm) with features suggestive of LVNC (Fig. 1) and normal LV systolic and diastolic function (LV ejection fraction of 66%).
Two-dimensional echocardiogram in short-axis view at end-systole (left) and apical 5-chamber view at end-diastole (middle), with trabeculated myocardium mainly involving the left ventricular apex and septal and lateral wall. Intertrabecular spaces are perfused from the ventricular cavity, as visualized on color Doppler imaging (right).
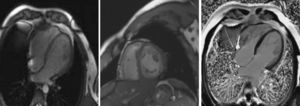
For additional morphological characterization, cardiac magnetic resonance (CMR) imaging was carried out (Fig. 2), further substantiating the diagnostic hypothesis of LVNC. No delayed-contrast hyperenhancement indicative of myocardial fibrosis was observed (Fig. 2, right).
Four-chamber (left) and short-axis (middle) steady-state free precession cine magnetic resonance image in end-diastole, confirming noncompaction of the left ventricle. Right: cardiac magnetic resonance four-chamber view after gadolinium administration, revealing no myocardial delayed enhancement.
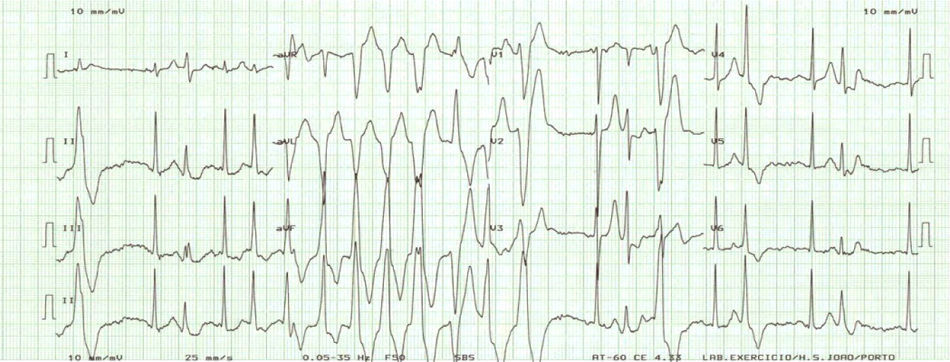
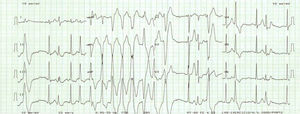
In order to evaluate the arrhythmic risk, 24-h ambulatory electrocardiographic (Holter) monitoring and an exercise stress test were scheduled. While the former did not show any abnormality, the latter disclosed exercise-induced nonsustained ventricular tachycardia (VT) (Fig. 3).
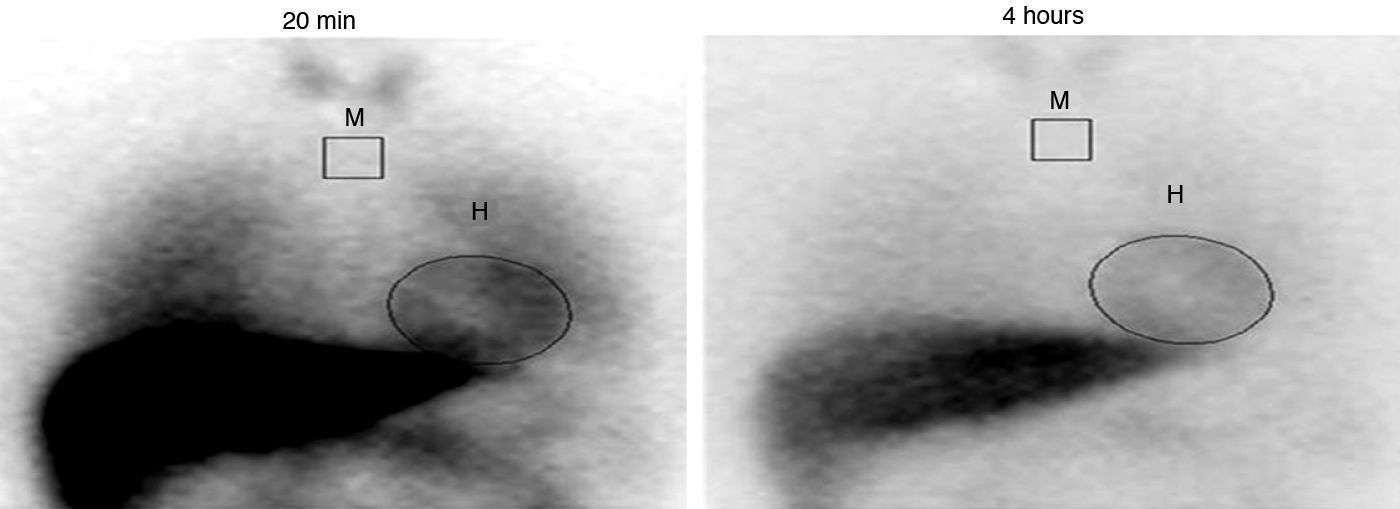
In an attempt to clarify the pathophysiology of this unusual cardiomyopathy, single-photon emission computed tomography (SPECT) imaging of the heart was performed, using 123I-MIBG. At that time, the patient was not taking any medication. While cardiac 123I-MIBG uptake was normal (late heart-to-mediastinum ratio [HMR] of 2.01), a significant increase in 123I-MIBG washout rate (WR) was observed (43.5%, vs the normal value <30%), suggesting cardiac sympathetic nervous dysfunction (Fig. 4).
Cardiac 123iodine-metaiodobenzylguanidine washout rate computed on planar images reflecting the percentage change in cardiac activity from early (20min, left) to late images (4h, right), according to the formula: washout rate=[((H–M) early−(H–M) late)/(H–M) early]×100 (%). H: heart; M: mediastinum.
Given the clinical and imaging findings in addition to the family history of SCD, an implantable cardioverter-defibrillator was implanted. In addition, beta-blocker therapy was instituted, despite the patient's normal LV systolic function and sinus bradycardia, in view of the presence of exercise-induced arrhythmia and cardiac adrenergic hyperactivity.
A neuromuscular assessment was scheduled as well as family screening. At two years of follow-up, the patient remains asymptomatic, without evidence of LV dysfunction.
DiscussionIsolated left ventricular noncompaction, albeit rare, is an increasingly recognized cardiomyopathy. The etiology of LVNC is still not definitely established. Premature arrest in the normal process of myocardial compaction during fetal ontogeny is the most widely acknowledged hypothesis.1,2 The prevalence reported in the largest series of adult patients with LVNC was 0.014%,3 but since this value may reflect selection bias, the true prevalence of this disease is still unknown. Presentation of LVNC involves a wide spectrum of clinical scenarios, including heart failure, thromboembolic events, supraventricular or ventricular arrhythmias, conduction disturbances, and even sudden death. Age at diagnosis is also variable, with cases identified during the prenatal period and in nonagenarian patients. Echocardiography is considered one of the main imaging modalities for diagnosis and the most frequently used echocardiographic criteria are those proposed by Jenni et al.4 and Stollberger et al.5 CMR imaging is increasingly recognized as an important technique in this field, providing information on myocardial morphology and tissue characteristics and also noninvasively documenting the presence and extent of myocardial fibrosis through delayed-enhancement sequences. Specific CMR criteria for the diagnosis of pathological LVNC have been recommended.6 Prognosis may be poor, especially for those who have symptoms of heart failure, a history of sustained ventricular tachycardia or an enlarged left atrium, with half of deaths occurring suddenly.3 One of the major implications of early detection of LVNC is the possibility of preventing SCD. However, identifying patients most at risk of death and most likely to benefit from currently available treatment remain a challenge. Specific factors for risk stratification of SCD in LVNC have not been fully identified. In addition to LV systolic dysfunction, other factors should be considered for identification of patients at high risk of SCD, such as nonsustained VT on 24-h Holter recording, positive family history, syncope or inducible VT/ventricular fibrillation.7
Impaired cardiac adrenergic innervation, as assessed by 123I-MIBG imaging, is strongly related to mortality in patients with HF, independently of its cause. Abnormalities in 123I-MIBG imaging have also been demonstrated in patients with more uncommon entities, such as syndrome X, Brugada syndrome and idiopathic ventricular fibrillation. We performed 123I-MIBG cardiac scintigraphy in order to elucidate the pathophysiology of LVNC. The most commonly used myocardial 123I-MIBG indices are HMR and myocardial WR. Late HMR combines information on neuronal function from uptake to release through the storage vesicle at nerve terminals, while WR is an index of sympathetic activity in terms of the ability to store norepinephrine. An increase in cardiac 123I-MIBG WR represents enhanced norepinephrine spillover from sympathetic nerve endings. A strong relationship between late HMR and occurrence of major cardiac events in heart failure patients has been consistently observed.8,9 This prognostic indicator was independent of LV ejection fraction. Besides late HMR, other 123I-MIBG parameters have also been reported as having prognostic value: in chronic heart failure patients, 123I-MIBG WR was also reported to be a powerful predictor of SCD in patients with mild to moderate chronic heart failure, independently of LV ejection fraction.10123I-MIBG imaging also provides independent prognostic information in patients with heart failure with preserved ejection fraction.11
Abnormally decreased 123I-MIBG uptake and increased WR have been observed in a patient with LVNC and severely reduced LV systolic function who suffered cardiac arrest.12 We now report the presence of abnormal cardiac sympathetic neurotransmission in a patient suffering from LVNC but presenting preserved systolic function. Late HMR is an index of sympathetic denervation and is usually altered with more severe heart failure, which may explain its normal value in our patient.
The presence of cardiac adrenergic dysfunction gives a new insight into the pathophysiology of LVNC, as this may be related to the increased arrhythmic risk observed in this unusual cardiomyopathy. This finding further supported the decision to implant an ICD, also in view of the patient's strong positive family history for SCD and exercise-induced nonsustained TV.
ConclusionsLeft ventricular noncompaction is a cardiomyopathy characterized by a distinctive morphological appearance of the LV myocardium. No definite information is currently available regarding its true etiology and natural history. Abnormal cardiac sympathetic neurotransmission seems to play a role in the pathophysiology of this entity, possibly explaining the increased arrhythmic risk. Nevertheless, further studies are needed in order to validate this hypothesis.
Conflicts of interestThe authors have no conflicts of interest to declare.



![Cardiac 123iodine-metaiodobenzylguanidine washout rate computed on planar images reflecting the percentage change in cardiac activity from early (20min, left) to late images (4h, right), according to the formula: washout rate=[((H–M) early−(H–M) late)/(H–M) early]×100 (%). H: heart; M: mediastinum. Cardiac 123iodine-metaiodobenzylguanidine washout rate computed on planar images reflecting the percentage change in cardiac activity from early (20min, left) to late images (4h, right), according to the formula: washout rate=[((H–M) early−(H–M) late)/(H–M) early]×100 (%). H: heart; M: mediastinum.](https://static.elsevier.es/multimedia/08702551/0000003100000003/v1_201308021306/S0870255112000030/v1_201308021306/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)


