Treadmill exercise testing has low specificity for the detection of significant epicardial coronary artery disease (CAD). A possible mechanism to explain some of the false positives is transient subendocardial ischemia induced by intraventricular gradients (IVG) during stress. The development of IVG during dobutamine stress echocardiography (DSE) occurs in 8-38% of non-selected populations.
ObjectivesTo determine:
- 1.
the prevalence of IVG in a selected population of false positives on treadmill stress testing;
- 2.
whether this prevalence is different from that described for non-selected populations;
- 3.
whether patient characteristics are related to the presence of IVG;
- 4.
the relation between the presence of IVG and the occurrence of ECG abnormalities, symptoms and blood pressure.
We evaluated 50 consecutive patients with false positive treadmill stress tests (normal CT coronary angiography, nuclear perfusion tests or angiography) with DSE (2D and Doppler evaluation). All DSE exams were negative for ischemia. Stress-induced IVG was seen in 34 of the 50 patients (68%) and 16 patients (32%) did not develop IVG (p<0.05). The prevalence of IVG in our selected population (68%) was significantly higher than that described for non-selected populations (8-38%) (p<0.001). Most patient characteristics (gender, age, risk factors for CAD, treatment with beta-blockers/calcium antagonists, significant valvular disease/left ventricular hypertrophy [LVH], symptoms, and blood pressure during stress) were not statistically associated with the prevalence of IVG (p>0.05). However, the presence of IVG was associated with the occurrence of ischemic ST depression during dobutamine stress echo (p<0.05).
Conclusions- 1.
The prevalence of IVG during dobutamine stress echocardiography in a selected population of false positives on treadmill stress testing is very high, occurring in more than two-thirds of patients.
- 2.
This prevalence is significantly higher than that described for non-selected populations.
- 3.
Age, gender, risk factors for CAD, treatment with beta-blockers/calcium channel antagonists, significant valvular disease/LVH, symptoms and blood pressure during stress were not associated with the presence or absence of IVG.
- 4.
The presence of IVG is associated with the occurrence of ischemic ST changes during dobutamine stress echocardiography.
A prova de esforço é um exame com baixa especificidade na detecção de doença coronária epicárdica significativa (DAC). Um mecanismo possível para explicar alguns dos seus falsos positivos (FP) é a isquémia subendocárdica transitória induzida pelos gradientes intraventriculares (GIV) durante o stress. O desenvolvimento de GIV durante o ecocardiograma de sobrecarga com dobutamina (ESD) ocorre, em populações não seleccionadas, em 8-38% dos doentes.
ObjectivosDeterminar:
- 1.
A prevalência de GIV numa população seleccionada de FP de prova de esforço.
- 2.
Se esta prevalência é diferente da descrita para populações não seleccionadas.
- 3.
Se as características dos doentes estão relacionadas com a presença de GIV.
- 4.
A relação entre a presença de GIV e a ocorrência de alterações electrocardiográficas, o aparecimento de sintomas e a pressão arterial.
O nosso estudo analisou 50 doentes consecutivos considerados falsos positivos de prova de esforço (Angio-TC coronária, Cintigrafia de Perfusão Miocárdica ou Angiografia normais) com avaliação por ESD. Todos os ESD foram negativos para isquémia. 34 dos 50 doentes (68%) apresentaram GIV induzido pelo stress e 16 doentes (32%) não desenvolveram GIV (p < 0,05). A prevalência de GIV na população seleccionada (68%) foi significativamente mais elevada que a descrita para populações não seleccionadas (8-38%) (p < 0,001). A maioria das características dos doentes (género, idade, factores de risco para DAC, tratamento com beta-bloqueantes/antagonistas dos canais de cálcio, doença valvular significativa/hipertrofia ventricular esquerda (HVE), sintomas, pressão arterial durante o stress) não foram estatisticamente relevantes para explicar a presença de GIV (p > 0,05). No entanto, a presença de GIV relacionou-se com a ocorrência de depressão do segmento ST durante o ecocardiograma de sobrecarga com dobutamina (p < 0,05).
Conclusões- 1.
A prevalência de GIV durante o ecocardiograma de stress com dobutamina em populações seleccionadas de falsos positivos de prova de esforço é muito elevada, ocorrendo em 2/3 dos doentes.
- 2.
Esta prevalência é significativamente mais alta que a descrita para populações não seleccionadas.
- 3.
Idade, género, factores de risco para DAC, terapêutica com beta-bloqueantes/antagonistas dos canais de cálcio, doença arterial significativa/HVE, sintomas e a pressão arterial durante a sobrecarga não se relacionam com a presença ou ausência de GIV.
- 4.
A presença de GIV está relacionada com a ocorrência de alterações isquémicas do segmento ST durante a ecocardiografia de sobrecarga com dobutamina.
Treadmill exercise testing is a standard exam for the detection of myocardial ischemia and ST-segment depression during exercise is a positive criterion for ischemia. However, this exam presents low sensitivity (68%) and low specificity (71%) for the detection of significant epicardial coronary artery disease.1 False positive tests are generally explained by mechanisms such as abnormal repolarization on the baseline ECG (low serum potassium levels, left ventricular [LV] hypertrophy [LVH], valvular disease and digitalis effect, among others) or excessive increase in blood pressure during exercise.2–4
Another possible explanation is the development of intraventricular gradients (IVG) during exercise.5 This phenomenon can be identified during stress echocardiography,6 a test with higher sensitivity (85%) and specificity (86%) for the detection of epicardial coronary artery disease (CAD).1 IVG develop during dobutamine stress echocardiography (DSE) in 8-38% of non-selected populations and are often described as having no clinical impact.7–15 However, the prevalence of IVG has never been assessed in a selected population of patients with positive treadmill exercise test and without significant CAD.
Objectives- 1.
To assess the prevalence of IVG during DSE in a selected population of patients with a false positive treadmill exercise test;
- 2.
to determine whether this prevalence is different from that described for non-selected populations;
- 3.
to determine whether patient characteristics (gender, age, risk factors for CAD, treatment with beta-blockers or calcium antagonists and significant valvular disease or LVH) are associated with the presence or absence of IVG;
- 4.
to assess the relation between the presence of IVG and the occurrence of ECG changes, symptoms and blood pressure response to stress.
We prospectively studied 50 consecutive patients (36 men, 14 women, aged 31-79 years, mean 55) followed in the outpatient clinic of the Cardiovascular Center of Hospital da Luz with the diagnosis of a false positive treadmill exercise test. These patients were defined as having a positive standard Bruce protocol treadmill exercise test by electrical criteria (horizontal or downsloping ST-segment depression of ≥1mm for at least 0.08seconds in two adjacent leads during exercise or recovery) but negative results in an anatomical technique (invasive coronary arteriography or invasive CT coronary angiography with coronary artery stenosis <50%) or in a nuclear functional study (no inducible ischemia). The selection of the anatomical versus functional test to confirm the result of the treadmill test was an individual decision by the consulting cardiologist.
In each patient we assessed general clinical data including gender, age, risk factors for CAD, treatment with beta-blockers or calcium channel antagonists and significant valvular disease or LVH. All of these 50 patients were referred for conventional echocardiography followed by DSE.
Standard echocardiographyAll the examinations were performed with the patient in left lateral decubitus position, using a Vivid 7 echocardiographic system (GE Vingmed Ultrasound AS, Horten, Norway) with a 1.7-3.5MHz phased-array transducer and harmonic imaging, using conventional echocardiographic views, stored digitally for subsequent analysis. All the echocardiographic measurements (M-mode, two-dimensional, blood pool and tissue Doppler echocardiography) were performed in accordance with the recommendations of the American Society of Echocardiography and the European Association of Echocardiography.16,17
In accordance with these recommendations, special care was taken to assess the presence and severity of LVH, systolic anterior motion (SAM) of the mitral valve and intraventricular gradients at rest.16,17
As patients’ filling status could affect intraventricular pressure gradients, LV diastolic function/filling pressures and right atrial pressure (inferior vena cava diameter and respiratory variation) were also assessed.16,17
Dobutamine stress echocardiographyThe general stress echocardiography protocol was as recommended by the European Association of Echocardiography18 and the standard dobutamine stress echo protocol was followed in all patients. Briefly, continuous intravenous infusion of dobutamine was begun with a dose of 5μg/kg/min and increased every 3min to 10, 20, 30 and 40μg/kg/min. Blood pressure and a 12-lead ECG were recorded at baseline, at the end of each stage of dobutamine infusion and during recovery. We also recorded the symptoms reported during the test and classified the ECG changes as with or without positive criterion for myocardial ischemia. If the target heart rate was not achieved at the peak of dobutamine infusion, intravenous atropine (0.5 up to 2.0mg) was added.
Besides the standard assessment of new wall motion abnormalities (diagnostic of myocardial ischemia), we also assessed the development of SAM and IVG in the left ventricular outflow tract (LVOT) during the exam. The examination was performed from multiple imaging planes, with the transducer carefully angulated to achieve maximum velocity across the LVOT. Particular care was taken to isolate a spectral Doppler profile showing a relatively slow increase in velocity and culminating in delayed peak velocity in mid-systole, a characteristic waveform of LVOT obstruction. Gain and filter settings were adjusted to obtain the signal with the highest audible frequency, maximal peak velocity, and optimal signal-to-noise ratio. The LVOT signal was distinguished from that of mitral regurgitation by direct visualization of color Doppler flow on the two-dimensional images and by recognizing that mitral regurgitation jets are characterized by their earlier onset, more abrupt initial increase in velocity and higher peak velocity than outflow tract gradient waveforms. Midventricular gradients were also carefully excluded by confirming that the recorded peak velocity had its origin in the LVOT (aliasing and SAM in the outflow tract) and not in other areas of the LV cavity (absence of color aliasing near the apex).
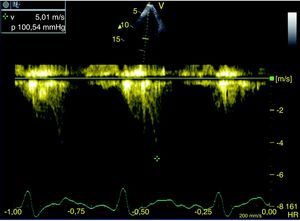
Doppler recordings were reviewed independently and randomly by two blinded observers, who only analyzed beats with a Doppler spectral envelope that was assumed to represent the velocities across the LVOT and for which there was sharp and unambiguous definition of the entire waveform contour. Post-ectopic beat waveforms were rejected. The Doppler beam was assumed to be almost parallel to systolic flow in the LVOT and therefore no angle correction was used in the estimation of the pressure gradient. LVOT pressure gradient was estimated by the modified Bernoulli equation (P=4V2, where P is the pressure gradient in mmHg and V is the maximum flow velocity in m/s. For each study, the mean of the three highest velocity beats was obtained. A significant intraventricular gradient was defined as a new peak gradient of 30mmHg during DSE, with a late systolic peak (Figure 1), with simultaneous SAM and outflow tract evidence of systolic aliasing on color Doppler.
Statistical analysisCategorical variables were expressed as percentages and studied using the chi-square test. To determine whether patient characteristics were associated with the presence of IVG and to relate the presence of IVG with the occurrence of ECG changes or symptoms, the statistical analysis program SPSS version 18.0 was used. These results were considered significant if p<0.05.
To compare the selected population with non-selected populations a binomial distribution was used. For this analysis a p-value of <0.001 indicates statistical significance.
ResultsThe clinical data of 50 patients included in the study are shown in Table 1. The population included 36 men and 14 women, aged between 31-79 years (mean 55) with a mean body mass index of 24.8. In this population only 18% of patients were asymptomatic; 66% presented fatigue, 32% had typical chest pain and 42% presented atypical chest pain. According to our data, 78% of the patients had risk factors for CAD: 24% had one risk factor, 36% two risk factors and only 4% (two patients) had three risk factors. Only three patients were under treatment with beta-blockers and none were taking calcium channel antagonists (Table 1).
Clinical data of the 50 patients in our study.
| Patient | Gender | Age | CAD risk factors | Treatment |
| 1 | F | 42 | S | No |
| 2 | F | 74 | DM; D | No |
| 3 | M | 31 | S | No |
| 4 | F | 51 | S; D; FH | No |
| 5 | M | 58 | D; HT | Atenolol |
| 6 | M | 40 | HT | No |
| 7 | M | 39 | D; FH | No |
| 8 | F | 65 | No | No |
| 9 | M | 58 | D | No |
| 10 | M | 56 | S; D | No |
| 11 | M | 44 | No | No |
| 12 | F | 54 | No | No |
| 13 | M | 57 | HT; D | No |
| 14 | M | 64 | No | No |
| 15 | M | 57 | HT; D | No |
| 16 | F | 56 | No | No |
| 17 | F | 54 | DM; D; HT | No |
| 18 | M | 53 | HT; D | No |
| 19 | M | 42 | No | No |
| 20 | M | 62 | S; D | No |
| 21 | M | 51 | No | No |
| 22 | M | 64 | HT | No |
| 23 | M | 51 | HT; FH | No |
| 24 | M | 62 | HT | No |
| 25 | M | 58 | No | No |
| 26 | F | 61 | D; HT | No |
| 27 | M | 55 | No | No |
| 28 | F | 78 | D; HT | No |
| 29 | M | 58 | D; HT | No |
| 30 | M | 38 | No | No |
| 31 | M | 39 | No | Bisoprolol |
| 32 | M | 51 | D | No |
| 33 | F | 60 | D; FH | No |
| 34 | F | 56 | D; DM | No |
| 35 | M | 49 | D; FH | No |
| 36 | F | 49 | No | No |
| 37 | M | 39 | HT | Bisoprolol |
| 38 | M | 59 | HT; FH | No |
| 39 | M | 44 | No | No |
| 40 | M | 43 | HT; FH | No |
| 41 | M | 35 | No | No |
| 42 | M | 64 | S | No |
| 43 | F | 51 | D | No |
| 44 | M | 65 | No | No |
| 45 | M | 64 | No | No |
| 46 | F | 53 | No | No |
| 47 | M | 44 | HT | No |
| 48 | M | 62 | HT; FH | No |
| 49 | M | 79 | HT | No |
| 50 | M | 63 | No | No |
M: male; F: female; S: smoking; DM: diabetes mellitus; D: dyslipidemia; HT: hypertension; FH: family history; MR: mitral regurgitation; LVH: left ventricular hypertrophy.
Table 2 shows the results of the ECG, conventional echocardiogram and dobutamine stress echo.
ECG, conventional echocardiography and dobutamine stress echocardiography data.
| Patient | Baseline ECG | Baseline echo | Dobutamine stress echo | ||
| IVG | Symptoms | ST changes | |||
| 1 | Normal | Normal | No | No | No |
| 2 | Normal | Normal | No | No | No |
| 3 | LVH | Normal | No | No | Yes |
| 4 | Normal | MVP, mild MR | No | No | No |
| 5 | Normal | Normal | No | No | No |
| 6 | Normal | Normal | No | No | No |
| 7 | Normal | Normal | No | No | No |
| 8 | Normal | Normal | No | No | No |
| 9 | Normal | Normal | No | No | No |
| 10 | Normal | Normal | No | No | No |
| 11 | Normal | Normal | No | No | No |
| 12 | Normal | Normal | No | No | No |
| 13 | Normal | Normal | No | No | No |
| 14 | Normal | Normal | No | No | No |
| 15 | Normal | Normal | No | No | No |
| 16 | Normal | Normal | No | No | No |
| 17 | Normal | Normal | 50 | Yes | Yes |
| 18 | Normal | Mild AS | 50 | No | No |
| 19 | Normal | Normal | 50 | No | No |
| 20 | Normal | Normal | 50 | No | No |
| 21 | Normal | Normal | 54 | No | No |
| 22 | Normal | Normal | 60 | No | Yes |
| 23 | Normal | MVP | 80 | Yes | No |
| 24 | Normal | Normal | 100 | No | No |
| 25 | Normal | Normal | 80 | No | No |
| 26 | Normal | Normal | 80 | No | Yes |
| 27 | Normal | Normal | 80 | No | No |
| 28 | Normal | Mild AS | 80 | No | Yes |
| 29 | Normal | Normal | 80 | No | No |
| 30 | Normal | Normal | 100 | No | Yes |
| 31 | Normal | Normal | 100 | No | Yes |
| 32 | Normal | Normal | 100 | No | No |
| 33 | Normal | Normal | 100 | No | Yes |
| 34 | Normal | Normal | 100 | No | No |
| 35 | Normal | Normal | 100 | No | No |
| 36 | Normal | Normal | 100 | No | Yes |
| 37 | Normal | LVH | 100 | Yes | Yes |
| 38 | Normal | Normal | 100 | No | Yes |
| 39 | Normal | Normal | 104 | No | Yes |
| 40 | Normal | Normal | 110 | No | No |
| 41 | Normal | Normal | 110 | No | Yes |
| 42 | Normal | Normal | 110 | No | Yes |
| 43 | Normal | Normal | 115 | No | Yes |
| 44 | Normal | Normal | 115 | No | Yes |
| 45 | Normal | Normal | 120 | No | No |
| 46 | Normal | Normal | 130 | No | Yes |
| 47 | Normal | Normal | 130 | No | Yes |
| 48 | Normal | Normal | 135 | No | Yes |
| 49 | Normal | Normal | 180 | No | No |
| 50 | Normal | Normal | 200 | No | Yes |
LVH: left ventricular hypertrophy; MVP: mitral valve prolapse; MR: mitral regurgitation; AS: aortic stenosis; IVG: intraventricular gradient, mmHg.
The baseline ECG was normal in all but one patient (patient 3), who presented LVH (Sokolow-Lyon index 37mm). The standard echocardiogram (performed before the dobutamine stress echo) also did not show relevant abnormalities in most patients (Table 2). Remarkably, in all patients the LVOT diameter was normal; none of the patients had SAM or LVOT gradient at rest; none of the patients had septal hypertrophy and only one (patient 37) showed concentric LVH (LV mass index 141g/m2, relative wall thickness 0.46, interventricular septum thickness/posterior wall thickness 1.1); and in all patients LV cavity dimensions and volumes were normal, as were conventional indices of global systolic function. Estimation of LV filling pressures, as well as of right atrial pressure (inferior vena cava diameter and respiratory variation), showed normal results in all patients.
During dobutamine stress echocardiography none of the 50 analyzed patients developed echocardiographic signs of myocardial ischemia and the dobutamine stress test was considered negative for ischemia in all of them. However, 34 patients (68%) developed a new IVG in the LVOT, while the other 16 (32%) had no stress-induced IVG (p<0.05). The mean gradient developed during DSE was 99.32mmHg, ranging between 50-200mmHg (Table 2). The prevalence of IVG in our study (68%) was significantly higher than that described for non-selected populations (8-38%) (p<0.001).
We also evaluated whether certain factors (gender, age, risk factors for CAD, and significant valvular disease or LVH), usually considered predictors of the development of IVG, were associated with their development during DSE. According to our data, none of these factors was statistically associated with the development of IVG (p>0.05 for all of them). Moreover, we assessed the relation between new IVG and the occurrence of symptoms (dyspnea or angina) and blood pressure levels during DSE. We found that only three patients had symptoms, and no patient had hypotension, during DSE. Therefore neither the occurrence of symptoms nor blood pressure levels showed any significant relation with the development of LVOT gradients (p>0.05) during DSE.
However, the occurrence of ECG changes with positive criterion for myocardial ischemia during DSE was significantly associated with the development of IVG (p<0.05).
DiscussionThe presence of ventricular gradients during DSE has traditionally been considered a dobutamine-specific effect, with no physiological or clinical significance,19 and not reproducible during physical exercise.7,20
Our study shows that, in a selected population of false positives on treadmill stress testing, a very large proportion of patients (more than two-thirds) develop IVG during DSE. Though this prevalence (68%) is significantly higher than that described for non-selected populations (8-38%), the reasons for this difference and a plausible mechanism to explain it are not easy to find.
However, taking into account the results of our study, it seems reasonable to presume that the two phenomena – ST depression in the treadmill exercise test and IVG during DSE – may be linked, perhaps by a cause-effect relation, because during DSE the presence of IVG was also associated with ST-segment depression.
So, in our opinion, the development of intraventricular gradients during stress may not be only a dobutamine-specific effect but may also occur during exercise, which would explain the results of the treadmill exercise tests. The development of IVG during stress (dobutamine or exercise) would increase afterload, with subsequent pressure overload within the LV cavity and increased wall stress. This increased wall stress would decrease subendocardial perfusion, with ECG changes characteristic of subendocardial ischemia21–25 and in some cases with symptoms of dyspnea or chest pain.26
Thus, we propose that the intraventricular gradient itself causes subendocardial ischemia22,27,28 with ST-segment depression, both in the treadmill stress test and during DSE, without new wall motion abnormalities.
Some unexpected findings of our study are the lack of a significant relationship between induced outflow tract gradients and symptoms and blood pressure reduction during DSE. However, the decubitus (non-orthostatic) position of patients during DSE (helping to maintain adequate preload and cardiac output) and the typical late systolic occurrence of these gradients (when most of the LV stroke volume has been ejected from the left ventricle into the aorta) may explain these results.
Finally, the lack of a significant relation between the conventional predictors of IVG29–31 and the development of LVOT gradients in our study will need to be confirmed in future studies.
Our previous explanation for the association between IVG and ST depression in the exercise test, though conceptually attractive, should be confirmed in clinical settings, with patients undergoing exercise stress echocardiography to assess IVG before, during and after exercise.32,33 This will show whether the IVG induced during DSE is reproducible during exercise, and if so, it will confirm our theoretical explanation for the ST depression. Preliminary and still unpublished results from our center suggest that dobutamine-induced IVG are in some cases reproducible during exercise, though the magnitude of the gradient is lower than with dobutamine.
The reasons for this quantitative difference undoubtedly lie in the fact that dobutamine and exercise share some common features but also have different hemodynamic effects. Though they both have positive chronotropic and inotropic effects,34 dobutamine induces peripheral vasodilatation,27 while physical exercise induces vasodilatation of the arteries of the exercising muscular territory but visceral arterial vasoconstriction, which may prevent the development of IVG.7,20 On the other hand, orthostatism itself leads to a decrease in venous return and preload with a subsequent increase in intraventricular gradients, mainly during the recovery period (when the muscular pump of the lower limbs is absent, no longer preventing the decrease in preload).35
Finally, the decision to perform another diagnostic test after a negative scintigraphic study or after CT coronary angiography without significant stenosis was taken because most of our patients were still symptomatic and without diagnosis after these tests. As the sensitivity of these methods is not 100% we felt it clinically appropriate to perform another exam that is inexpensive, non-invasive and radiation-free and that could not only rule out ischemia but also provide another explanation for the clinical and treadmill test findings: dobutamine stress echocardiography.
LimitationsOur definition of a false positive treadmill exercise test, based on the normality of different tests (anatomical and functional), contributed to the non-homogeneity of the false positive population and is a limitation of this study. Future studies on this topic should define false positive patients based only on a single functional or anatomical test (even though none of them has 100% sensitivity and specificity).
Another limitation lies in the fact that we did not study a control group of individuals with negative exercise treadmill test in order to compare the proportion of patients with IVG in these patients with those with positive exercise test. However, we used the figures of non-selected populations taken from a number of studies in the literature.7–15
Finally, and as discussed above, exercise echocardiography should be performed in these patients, to assess the reproducibility of our results under physiological conditions.
ConclusionThis study shows that LV outflow tract gradients during dobutamine stress echocardiography in a selected population of false positives of treadmill stress test occur in more than two-thirds of patients, a significantly higher prevalence than that described in non-selected populations.
The presence of these gradients is associated with the occurrence of ischemic ST-segment changes, but not with symptoms or with blood pressure reduction.
The development of intraventricular pressure gradients during stress may therefore explain some of the false positive results of treadmill exercise tests. To confirm this theory, our results must be reproducible in physiological conditions, during exercise stress echocardiography.
Conflicts of interestThe authors have no conflicts of interest to declare.