Infective endocarditis is a microbial infection of the endocardium and it is rare in the pediatric population. In children, congenital heart disease is one of the most important risk factors for developing infective endocarditis and can involve other structures in addition to cardiac valves. The prognosis is generally better than in other forms of endocarditis, although the average mortality rate in the pediatric population is 15-25%. Clinical manifestations can mimic other diseases such as meningitis and collagen-vascular disease or vasculitis. Therefore, a high degree of suspicion is required to make an early diagnosis. Gram-positive bacteria, specifically alpha-hemolytic streptococci, Staphylococcus aureus and coagulase-negative staphylococci, are the most commonly involved bacteria. Diagnosis is based on the modified Duke criteria, which rely mostly on clinical assessment, echocardiography and blood cultures. Antibacterial treatment should ideally be targeted. However, if no specific bacteria have been identified, patients should promptly be treated empirically with multiple drug regimens based on local resistance and the most common etiologies.
The authors describe a case of a seven-year-old girl with classic clinical signs of endocarditis, with a clinical twist.
A endocardite infecciosa é uma infecção microbiana do endocárdio e é rara na população pediátrica. Nessa faixa etária, a doença cardíaca congénita é um dos fatores de risco mais importantes para o desenvolvimento de endocardite infecciosa, pode envolver outras estruturas para além das válvulas cardíacas. O prognóstico é geralmente melhor do que noutras formas de endocardite, embora a taxa média de mortalidade em população pediátrica seja em torno a de 15-25%. As manifestações clínicas podem mimetizar outras doenças, tais como meningite, vasculite ou doença vascular do colagénio, e, por conseguinte, é necessário um elevado grau de suspeita para fazer um diagnóstico precoce. Os microrganismos mais frequentemente implicados são as bactérias gram-positivas, especificamente os estreptococos alfa-hemolíticos, Staphylococcus aureus e estafilococos coagulase-negativos. O diagnóstico baseia-se nos critérios de Duke modificados, que dependem principalmente da avaliação clínica, da ecocardiografia e de hemoculturas. O tratamento antibacteriano deve ser orientado ao microrganismo em causa. No entanto, na ausência de organismos identificados, o tratamento empírico não deve ser adiado, devem-se usar diferentes combinações de antibióticos e ter em conta as resistências locais e etiologias mais frequentes. Os autores descrevem um caso de uma menina de sete anos com sinais clínicos clássicos de endocardite, com uma reviravolta clínica.
Infective endocarditis (IE) is a microbial infection of the endocardium and it is rare in the pediatric population. In children, congenital heart disease is one of the most important risk factors for developing IE1 and can involve other structures in addition to cardiac valves. The prognosis is generally better than in other forms of endocarditis, although the average mortality rate in the pediatric population is 15-25%. Clinical manifestations can mimic other diseases such as meningitis and collagen-vascular disease or vasculitis. Therefore, a high degree of suspicion is required to make an early diagnosis. Gram-positive bacteria, specifically alpha-hemolytic streptococci, Staphylococcus aureus and coagulase-negative staphylococci, are the most commonly involved bacteria.2,3 Diagnosis is based on the modified Duke criteria, which rely mostly on clinical assessment, echocardiography and blood cultures. Antibacterial treatment should ideally be targeted. However, if no specific bacteria have been identified, patients should promptly be treated empirically with multiple drug regimens based on local resistance and the most common etiologies.
The authors describe a case of a seven-year-old girl with classic clinical signs of endocarditis, with a clinical twist.
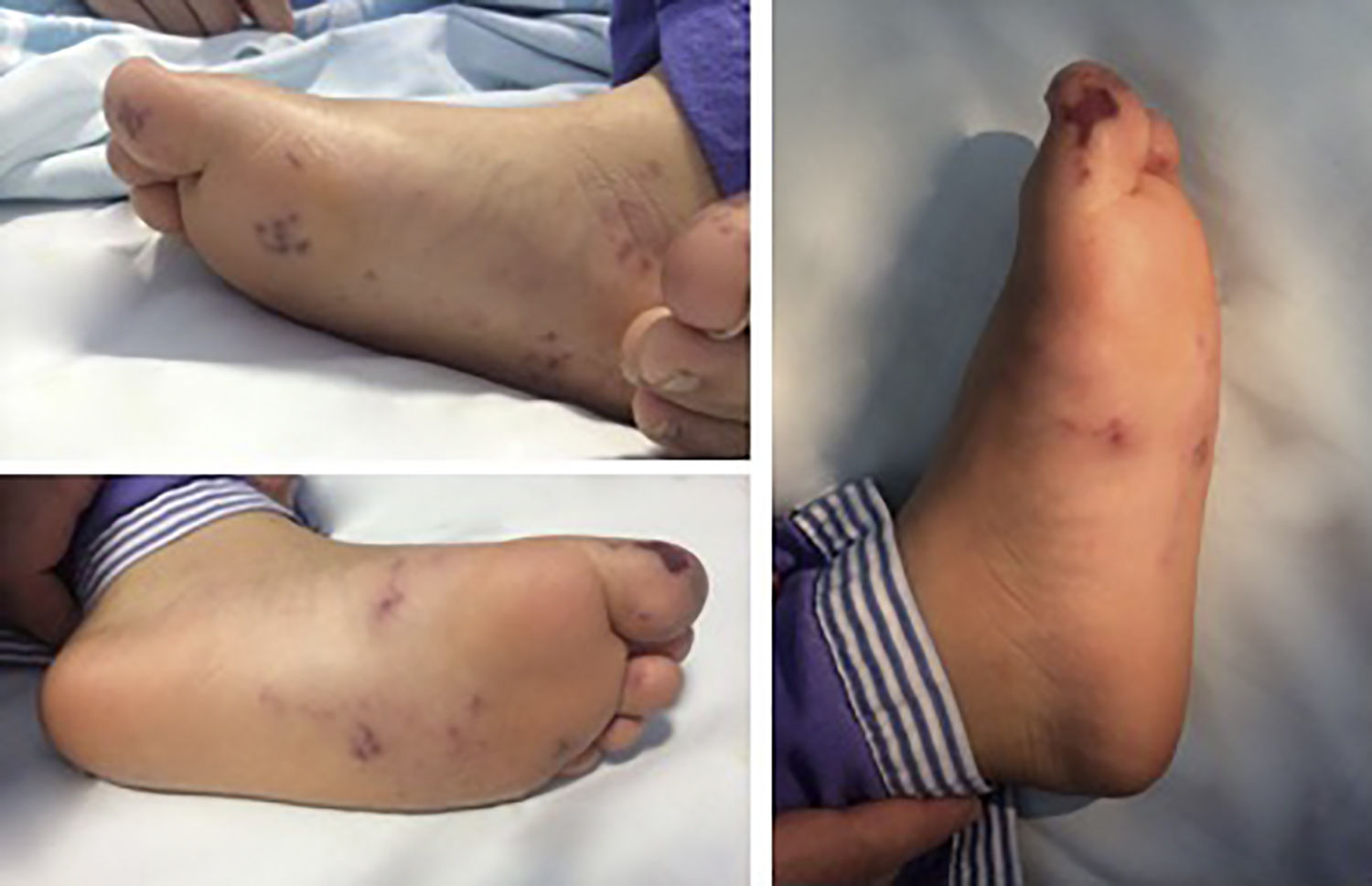
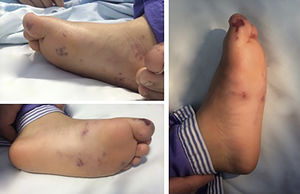
Case reportThe authors present the case of a seven-year-old girl admitted to the emergency room with a three-day history of acute onset abdominal pain, fever and recent-onset purpuric rash. On admission she was restless, tachycardic, hypertensive and had abdominal distension and hyperesthesia. A purpuric rash was also evident and limited to the buttocks, lower limbs and feet, and she also presented with tender bilateral edema (Figure 1). Initial blood tests showed leukocytosis, elevated acute-phase reactants, mild anemia, abnormal renal function, thrombocytopenia, and abnormal thromboplastin and prothrombin time. Urinalysis revealed the presence of hematuria and nephrotic proteinuria.
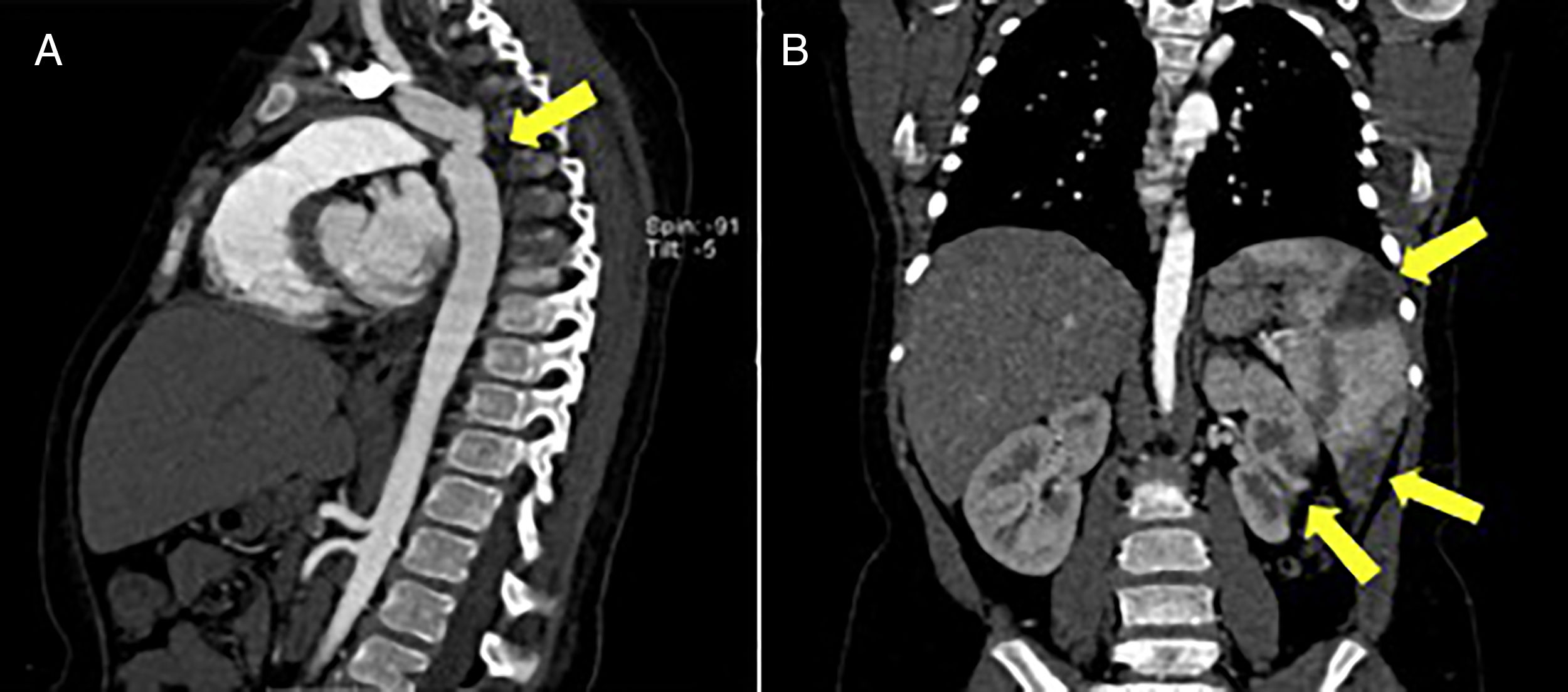
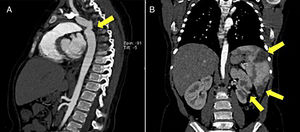
Blood cultures were performed and ceftriaxone was started empirically. Abdominal ultrasound showed splenomegaly as well as small-to-medium sized hypoechogenic lesions in the spleen, liver and both kidneys, compatible with thromboembolic infarcts. Due to possible infective embolic disease she was referred for cardiac assessment.
On examination, a low-grade (II/VI) systolic murmur was detected in the lower left sternal border, which radiated to the back. Echocardiogram revealed coarctation of the aorta at the isthmus with a systolic gradient of 60 mmHg with diastolic run-off. There appeared to be a movable filament-like structure adhering to the coarcted isthmus. No valvular or intracardiac vegetations were found. In order to clarify these findings, a transesophageal echocardiogram (TOE) was carried out, which showed the presence of a hyperechogenic movable “spur” at the aortic isthmus. A presumptive diagnosis of infective aortic endarteritis complicated by systemic embolization, hypertension and acute renal insufficiency was made. Vancomycin, titrated to the glomerular filtration rate, was added to the antibiotic regimen.
Staphylococcus schleiferi was later identified as the causative agent of the infective endarteritis. This organism is easily mistaken for Staphylococcus aureus. Antibacterial sensitivity testing showed that this agent was more sensitive to floxacillin and, as such, the latter was added to the therapeutic regimen and vancomycin was stopped. The patient became apyretic on day 5 post-admission and subsequent blood cultures were negative.
Blood pressure control was achieved with bisoprolol and nifedipine, after which the transcoarctation systolic gradient was reduced to 40 mmHg.
At week 2 post-admission, the clinical course was complicated by sudden onset of gastrointestinal (GI) bleeding (Hb 7.4 g/dl) leading to disseminated intravascular coagulopathy, requiring plasma and red blood cell transfusions. Normalization of hematological parameters was eventually achieved, and total parenteral nutrition was required for 10 days. Despite adequate diuresis under furosemide and spironolactone, worsening renal function ensued, which was characterized by high creatinine levels, slightly raised urea levels, nephrotic proteinuria and hematuria, and hypoalbuminemia. This was managed with prednisolone and, by week 3, renal function improved and normalized. The patient completed a total of six weeks of intravenous floxacillin and was discharged home on prednisolone, bisoprolol and nifedipine.
Additional imaging studies such as abdominal ultrasound, Computerized Tomography and Magnetic Resonance scans were performed (Figure 2).
DiscussionIn children, aortic endarteritis is an exceedingly rare condition even in the context of congenital heart disease, particularly as a source of systemic embolization, as was the case with our patient. Due to its rarity, no precise incidence is known, with very few cases found in literature. As is the case with endocarditis, endarteritis is a severe complication in a child with a structural heart defect where the mortality rate is <10% if the diagnosis is made early or approximately 25% if made at a later stage. Mortality is mostly due to congestive heart failure or systemic embolization complications.
The authors wish to highlight this rare form of endarteritis and, in particular, in the context of systemic embolization resulting in multiple organ failure. In this case, endarteritis at the coarctation site explained why systemic embolization was limited to the lower part of the body, causing a lower limb purpuric rash, renal insufficiency and GI bleeding. As congenital heart disease is a risk factor for endocarditis, it is imperative to rule out cardiovascular etiology for the embolic phenomenon.
To our knowledge, this is the first reported case of Staphylococcus schleiferi endarteritis originating at the site of a native aortic coarctation.4
Conflicts of interestThe authors have no conflicts of interest to declare.