A 49-year-old woman was hospitalized for acute left foot arterial ischemia. Arterial Doppler revealed occlusion of the dorsalis pedis and posterior tibial arteries. A computed tomography angiography performed to assess abdominal pain showed hepatic, splenic, renal and pancreatic infarctions. A splenic artery embolism and a small aortic wall thrombus at the celiac trunk were identified. No radiological signs of aortic atherosclerosis were found. No predisposing conditions for secondary aortic thrombosis or intracardiac embolic sources were detected. It was determined that primary aortic thrombosis, a rare though potentially serious condition, was to blame. Isolated aortic mural thrombosis therapy is not well established, although systemic anticoagulation, thrombolysis, thromboaspiration, endovascular stent grafting and surgical thrombectomy have been attempted with varying success. In our patient, systemic anticoagulation therapy was initiated and resulted in aortic thrombus resolution. Close clinical follow-up is crucial, as the aortic thrombus can recur despite anticoagulation and aggressive control of the atherosclerotic risk factors.
Doente de 49 anos do sexo feminino, admitida por isquemia aguda do pé esquerdo. O Doppler arterial revelou oclusão das artérias dorsal do pé e tibial posterior. Uma angiotomografia computorizada efetuada para avaliação de dor abdominal evidenciou enfartes hepáticos, esplénicos, renais e pancreático. Identificava-se êmbolo da artéria esplénica e pequeno trombo parietal aórtico ao nível do tronco celíaco; não existiam sinais radiológicos de aterosclerose da aorta. Não foram detetadas condições predisponentes para trombose secundária da aorta ou fontes embolígenas intracardíacas. Concluiu-se assim tratar-se de uma trombose primária da aorta, uma entidade rara, mas potencialmente grave. A terapêutica da trombose aórtica mural isolada não está bem estabelecida, embora anticoagulação sistémica, trombólise, tromboaspiração, stent endovascular e trombectomia cirúrgica estejam descritas com graus variáveis de sucesso. No nosso caso, foi feita anticoagulação sistémica com resolução do trombo aórtico. O follow-up clínico apertado é fundamental, uma vez que, apesar da anticoagulação e do controlo agressivo dos fatores de risco ateroscleróticos, a trombose aórtica pode recorrer.
Arterial embolism is a relatively common clinical disorder. Over 80% of all arterial emboli originate in the heart due to functional or structural conditions. Aortic thrombosis, a less common source of arterial embolism, is usually associated with aortic diseases such as extensive atherosclerosis, aneurysms, dissection, trauma, infections and tumors. Aortic thrombosis occurring in an apparently normal aorta is a very rare condition, and is referred to as primary aortic thrombosis. The etiology of thrombus formation in a macroscopically normal aorta is not well understood and has been associated with many disorders, particularly malignant neoplasms, hypercoagulation disorders, iatrogenic pharmacotherapy, recreational drug abuse and inflammatory diseases. We report the case of a woman with extensive arterial embolic events originating from a presumed primary aortic thrombus.
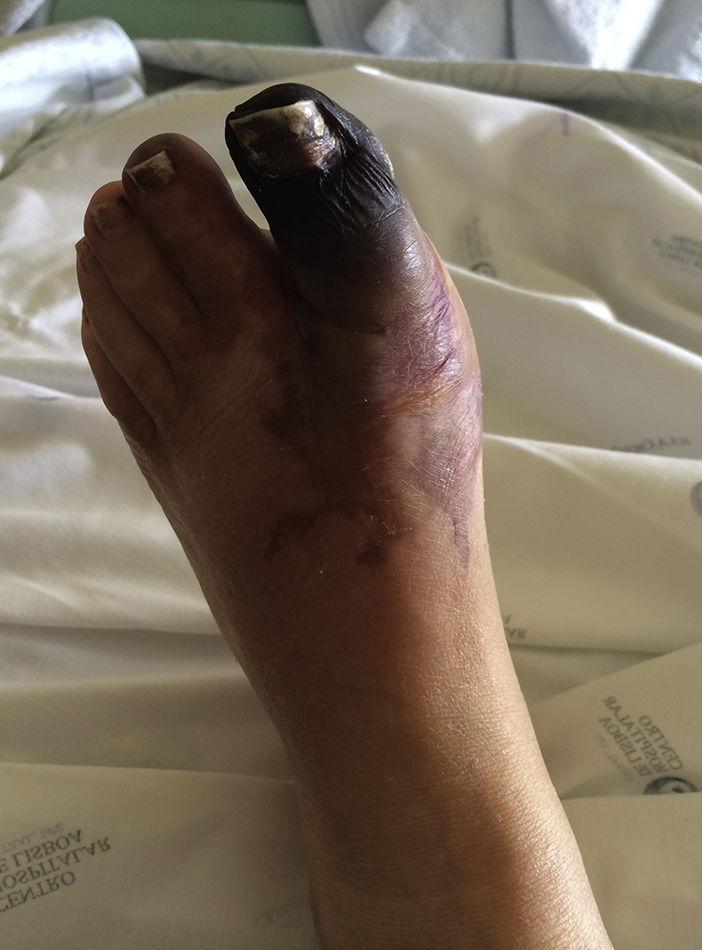
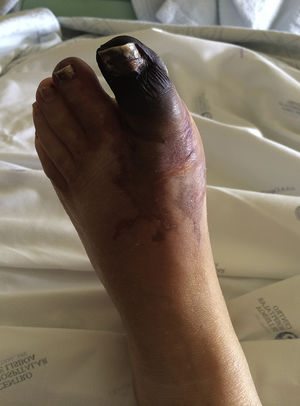
Case ReportA 49-year-old woman was admitted to the emergency room of her local hospital complaining of a 2-day history of bluish discoloration and pain in the left hallux (Figure 1). An acute arterial ischemia of the left foot was assumed, and she was transferred to our hospital for further assessment and eventual vascular examination.
She reported a 2-week history of dull epigastric pain with dorsolumbar irradiation, anorexia, nausea and sporadic postprandial vomiting.
She had a history of poorly controlled hypertension, had been on oral contraception for the last 20 years and had had menorrhagia for the last year. There was no other relevant history, such as hypercholesterolemia, diabetes or smoking habits. Clinical examination found elevated blood pressure (190/90mmHg), black-bluish discoloration of the left hallux (in mummification process), second left toe and distal dorsal face of the foot (Figure 1). This area was cold and painful, and the dorsalis pedis artery pulse was absent. Abdominal palpation caused pain in the upper quadrants, with no signs of peritoneal irritation. During the emergency room assessment, the patient experienced mental confusion associated with a hypertensive crisis. No focal neurologic deficits or meningeal signs were evident, and this was a transient, non-recurring episode.
The patient was transferred to our medical ward five days after close observation and monitoring in the Intensive Care Unit (ICU).
Investigations- -
Blood analysis: RBC count 4.39×1012/l, hemoglobin 10 g/dl, Mean Corpuscular Volume (MCV) 61.1 fl; Mean Corpuscular Hemoglobin (MCH) 18.2 pg, Red Cell Distribution Width (RDW) 22.2%, WBC count 3.9×109/l (88% neutrophils), platelet count 763×109/l; C-reactive protein (CRP) 273.6 mg/l, sedimentation rate 73 mm/h, urea 82 mg/dl, creatinine 1.4 mg/dl, sodium 129 mEq/l, potassium 4.1 mEq/l, alanine aminotranspherase 324 IU/l, aspartate aminotranspherase 143 IU/l, alkaline phosphatase 139 U/l, creatine phosphokinase 957 U/l, amylase 185 U/l, D-dimer 1248 μg/l, lactate dehydrogenase 530 U/l, glycated hemoglobin 6.7%, occasional glycaemia on two separate occasions 259 and 235 mg/dl, total cholesterol 135 mg/dl, High Density Lipoprotein (HDL) cholesterol 20 mg/dl, Low Density Lipoprotein (LDL) cholesterol 74 mg/dl, triglycerides 198 mg/dl, iron 13 μg/dl, ferritin 122.6 ng/ml, transferrin 1.73 g/l.
- -
Thrombophilia studies: prothrombin time (PT) 12.9 s, activated partial thromboplastin time (aPTT) 34.9 s, lupic anticoagulant: negative, anti-phospholipid antibodies: negative, antithrombin 118%; activated S protein 155%; free S protein 67.5%; C protein 165%; resistance to activated protein C ratio 3.0 (negative), homocysteine 9.0 μmol/l, prothrombin mutation (G20210A): negative. Antinuclear antibodies (ANA), rheumatoid factor, anti-dsDNA and anti-SSA/B antibodies: negative; anti-PR3 and MPO antibodies: negative. Protein electrophoresis, C’3 and C’4 complement fractions within normal range. Tumor markers: alpha-fetoprotein, beta-2-microglobulin, CA 125, CA 15.3, CA 19.9, CEA and CA 72.4 within normal range.
- -
Blood cultures (3) and urine culture: negative.
- -
Arterial Doppler of the left leg: occlusion of the dorsalis pedis and posterior tibial arteries.
- -
Electrocardiogram: sinus rhythm, without ischemic signs
- -
Thoracic roentgenogram: without parenchymal abnormalities
- -
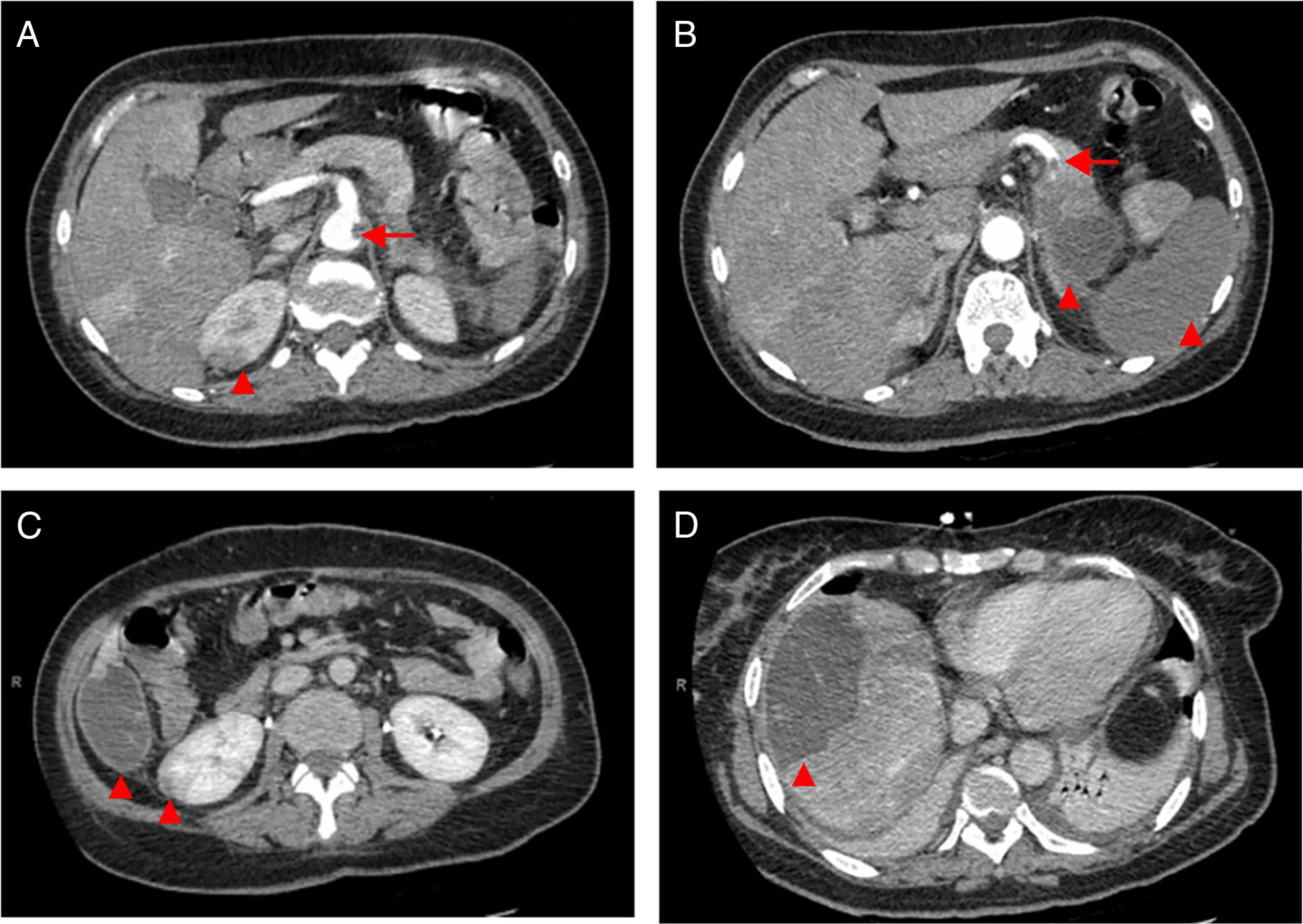
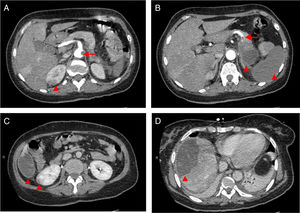
Computed tomography angiography (CTA) of the abdomen/pelvis (Figure 2A, 2B and 2C) at patient admission: complete splenic infarction without contrast enhancement except for a fine capsule; splenic artery occlusion. Hypodense ill-defined lesion in the pancreas tail measuring 55×38 mm with adjacent fat densification, but without contrast enhancement leading to a differential diagnosis between ischemic infarct and malignant neoplasm. Heterogeneous contrast enhancement of both kidneys, with triangular parenchymal areas of lower attenuation, suggestive of ischemic renal foci. Heterogeneous hepatomegaly with areas of non-contrast enhancement, the biggest one in segments VII and VIII; a similar area on the liver border and smaller hypodense areas that, in this context, could represent occlusive arterial conditions. Small parietal thrombus (9mm) in the left lateral aortic wall, in the axial plane of the celiac trunk, with no evident atheromatous lesions.
Figure 2.CT scan of the abdomen/pelvis showing total hypodensity of the spleen representing a complete infarct, without contrast enhancement except for a fine capsule. Splenic artery occlusion. Hypodense ill-defined lesion in the pancreas tail, measuring 55×38 mm with adjacent fat densification, but without contrast enhancement leading to a differential diagnosis between ischemic infarct and malignant neoplasm. Heterogeneous contrast enhancement of both kidneys, with triangular parenchymal areas of lower attenuation, suggestive of ischemic renal foci. Heterogeneous hepatomegaly with areas of non-contrast enhancement, the biggest one in segments VII and VIII; similar area on the liver border and smaller hypodense areas that, in this context, can represent occlusive arterial conditions. Small parietal thrombus (9mm) in the left lateral aortic wall, in the axial plane of the celiac trunk, with no evident atheromatous lesions.
- -
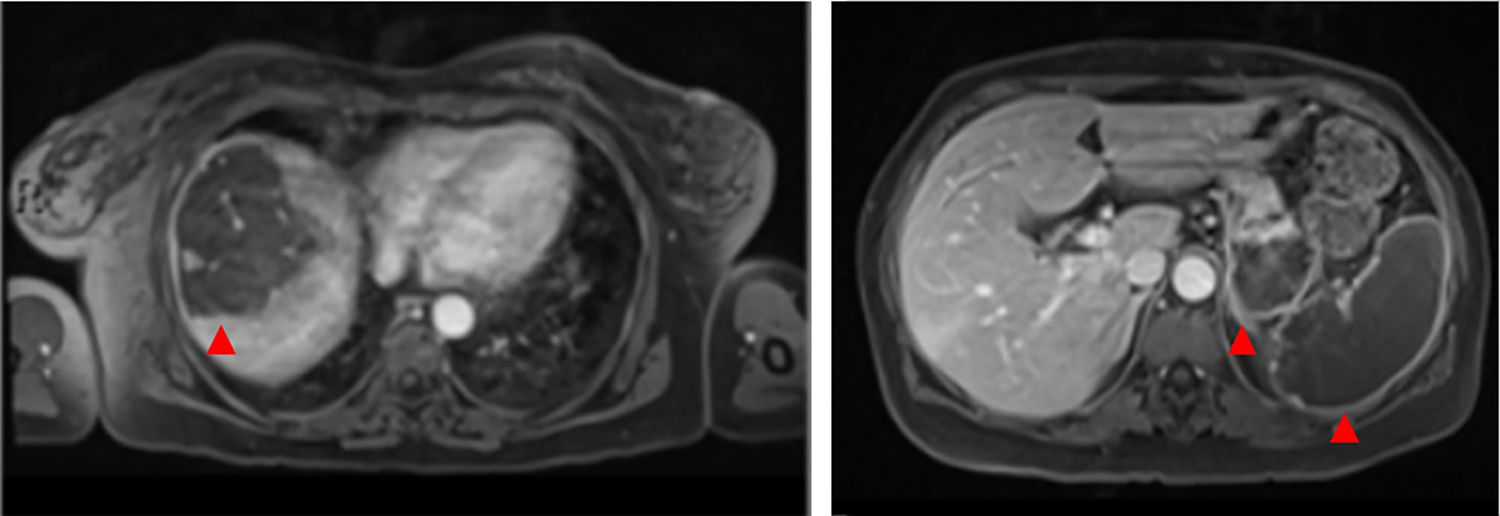
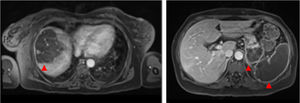
Magnetic resonance imaging (MRI) of the abdomen/pelvis (Figure 3A and 3B): multiple infarcts in the liver, kidneys and spleen, confirming also that the pancreatic lesion identified in the CTA represented ischemia.
- -
Cranial CT scan: multiple bilateral hypodense areas in subcortical topography, predominantly in the temporal, parieto-occipital white matter and corona radiata, without mass effect or hemorrhagic components. These images are compatible with small vessel vascular ischemia lesions.
- -
Cranial MRI: chronic ischemic microangiopathic leukoencephalopathy involving supratentorial subcortical and deep white matter, with hyperintense signal in the long TR sequences, without diffusion restriction (without acute vascular lesions). Diffuse cortico-subcortical cerebral atrophy, with subcortical predominance.
- -
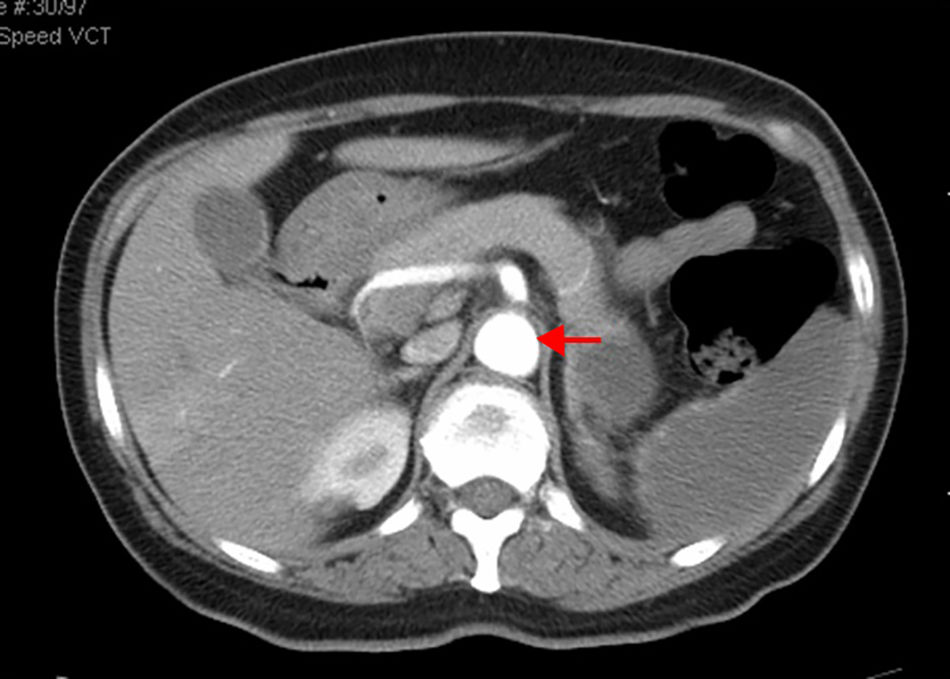
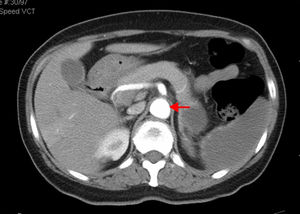
CTA of the abdomen/pelvis (Figure 4) at patient discharge: Abdominal aortic lumen patent throughout its trajectory, without identifiable thrombus. The ischemic clinical presentations described in the previous CT scan are identified, with better pancreatic ischemic lesion definition.
- -
Transesophageal echocardiography: left ventricular hypertrophy with preserved systolic function; valvular structures with no morphofunctional abnormalities. Left atrium and left ventricle not enlarged, with no endocavitary thrombus. Viewed thoracic aorta without lesions.
In the Intensive Care Unit, anticoagulation with unfractionated heparin was started in the first five days (initial bolus: 80 units/kg= 5000 U, followed by continuous infusion: 18 units/kg/h = 1000 U/h, titrated according to aPTT). In our medical ward, anticoagulation therapy was switched to low molecular weight heparin (enoxaparin 1 mg/kg b.i.d. = 60 mg b.i.d.). Warfarin was initiated before discharge with a target PT international normalized ratio (INR) of 2.5–3.5.
We also aimed to control cardiovascular atherosclerosis risk factors such as hypertension, hypercholesterolemia and diabetes. Blood pressure was controlled with perindopril 10 mg and amlodipine 10 mg q.d. During the patient's stay in our ward, glycemic control was achieved with rapid-acting insulin, according to the capillary blood glucose monitoring protocol.
Iron supplementation with ferrous sulphate 525 mg q.d. was started, and gynecologic examination to investigate abnormal menstrual bleeding was scheduled.
Vaccination against Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis was recommended.
After establishing the diagnosis and initial treatment, the patient was transferred back to her local hospital where she underwent left hallux amputation and continued oral anticoagulants. Six months after the initial event, the patient was asymptomatic, and no other new embolic events had occurred.
DiscussionThe foot lesion at presentation was compatible with an acute arterial ischemia from a probable embolic cause. Arterial Doppler of the left leg confirmed occlusion of the dorsalis pedis and the posterior tibial arteries. Endovascular intervention was not considered here because symptoms were present for longer than 48hours, mummification of the hallux was in progress and delimitation of the ischemic area was clear (Figure 1).
Although there were no clinical signs of acute abdomen, the pain in the upper quadrants could not be ignored. Moreover, blood test results (leukocytosis, elevated CRP and liver enzymes) were excessively abnormal, which could be explained only by the acute foot ischemia. Vascular events in the abdominal cavity, an infectious process (although there was no fever), neoplasm and pancreatitis had to be sought out. The CTA of the abdomen/pelvis showed an aortic wall thrombus in the axial plane of the celiac trunk and a complete splenic artery occlusion (Figure 2A and 2B). Multiple infarcts were present in some subsidiary arterial territories of the abdominal aorta: complete splenic infarct, renal ischemic foci, hepatic infarcts in distinct segments and a hypodense area in the pancreas tail leading to a differential diagnosis between pancreatic infarct and malignant neoplasm (Figure 2B, 2C and 2D). MRI of the abdomen/pelvis showed that the pancreatic lesion was an ischemic lesion and not a tumor (Figure 3A and 3B).
Tumor markers were negative, notwithstanding their limited value as diagnostic tools. The high levels of inflammatory parameters in the blood analysis (leukocytosis with neutrophilia, CRP) could represent a systemic inflammatory response to this extensive ischemic phenomenon. The absence of fever and negative blood and urine cultures favor this supposition and ruled out the hypothesis of an infectious process. It was interpreted as thrombocytosis due to functional asplenia.
The acute confusional state simultaneous to a hypertensive crisis led us to perform a CT scan to rule out cerebral stroke and hemorrhage. The CT scan images were compatible with small vessel vascular ischemic lesion. MRI confirmed there had been no recent ischemic events (thus excluding cerebral embolism) and supported the diagnosis of vascular leukoencephalopathy associated with poorly controlled hypertension.
Over 80% of all peripheral and visceral emboli originate in the heart from endocavitary thrombus associated with atrial fibrillation or post-infarct ventricular aneurysms, cardiac tumors (e.g. myxoma), endocarditis, paradoxical embolization and prosthetic heart valves. For differential diagnosis, a transesophageal echocardiogram was performed and ruled out an intracardiac embolic source.1 Continuous electrocardiographic monitoring during hospitalization in the ICU and serial electrocardiograms performed in our medical ward did not register atrial fibrillation or other dysrhythmias. The absence of intracardiac sources of embolism and the ischemic lesions limited to the abdominal viscera and lower limb suggested embolization from the descending aorta. The location of the aortic thrombus in the celiac trunk supports that it likely represents a remaining fragment of the major original thrombus that cleaved and embolized the infarcted areas. Somehow, we were lucky because this remaining identified thrombus was a diagnostic clue. If all the putative thrombotic mass had cleaved and migrated, establishing a diagnosis would have been more complicated.
Non-cardiac causes for aortic embolism include aortic thrombus associated with atherosclerosis and plaque rupture, aortic aneurysms, dissection, traumatic lesions and hypercoagulability.2,3 Examinations to rule out hypercoagulability were performed. Thrombosis in an apparently normal aorta (non-atherosclerotic, non-aneurysmal), despite being a rare condition, should also be considered, as was the case of our patient. This is usually referred to as primary aortic thrombosis, 4 a condition first described in 1958 by H. Gaylis in a 32-year-old man with thrombosis in the aortic bifurcation, without any obvious underlying atheromatous lesions.5 Few techniques were available at that time to identify the origin and etiology of this thrombus. Primary aortic thrombus in the literature is reported only by small series and isolated case reports.6–9 The thrombus is mostly located in the descending thoracic aorta, although presence of a thrombus in the aortic arch and abdominal aorta has been reported.10,11 The etiology of thrombus formation in a macroscopically normal aorta is not well understood, and has been associated with many disorders: cancer chemotherapy,12 cocaine intake,13 essential thrombocytopenia,14 some hypercoagulable states,15 heparin-induced thrombocytopenia,16 inflammatory bowel disease,17 acute pancreatitis,18 blunt trauma19 and aorta wall tumors,20 among others.21–23 The diagnosis of aortic thrombosis can be made using MRI, digitalized angiography or transesophageal echography in thoracic thromboses, but multi-slice computerized tomography (MSCT) is the initial investigation of choice.24 The equipment is widely available, and the examination is neither very expensive nor as invasive as angiography. The examination should be performed after intravenous injection of iodinated contrast material, which enables detecting thrombi, ischemic complications and possible causal lesions from the primary aortic thrombosis, such as malignant neoplasms.24
There is no robust evidence to offer guidance for the treatment of aortic mural thrombus. Most knowledge regarding this issue comes from isolated reports and somewhat extensive research of the literature. When considering therapeutic options for thoracic aorta thrombi, it is necessary to view them as a heterogeneous group rather than a single entity, each having a different clinical course and prognosis depending on its nature and etiology.8 Treatment modalities used with variable success for primary aortic thrombosis management include anticoagulation therapy alone, thrombolysis, thromboaspiration and surgery.10 Systemic anticoagulation with intravenous heparin followed by oral Coumadin derivatives has been considered the mainstay of therapy.25,26 Surgical thrombectomy is recommended for young patients, patients with suspected malignancy, in the presence of a large hypermobile thrombus or in recurrent embolic events, despite optimal anticoagulation.25 Endovascular stent grafting provides a minimally invasive therapeutic option, but its role and long-term outcome have not been established yet.27 Recently, the first successfully treated case of a symptomatic thoracoabdominal mural and floating intra-aortic thrombus was reported, using thoracic endograft, in conjunction with the AngioVac system (AngioDynamics, Latham, New York), a device that performs lysis and suction of the thrombus.28
Although the definition of primary aortic thrombosis implies a macroscopically “normal” aorta, we believe that a subclinical, non-identified premature atherosclerotic process may underlie the thrombus formation. Some atherosclerotic risk factors such as smoking, diabetes, hypertension and oral contraceptives have been associated with this “primary” aortic thrombosis, which is in consonance with our postulate.29,30 Our patient had a history of poorly controlled hypertension, had been on oral contraception for at least twenty years and had a possible pre-diabetes mellitus status aggravated by this stressful condition and pancreatic ischemia.
The patient was treated with systemic anticoagulation therapy and the identified remaining aortic thrombus resolved (Figure 4). When she was transferred back to her local hospital, we recommended long-term anticoagulation with Coumadin derivatives, target INR of 2.5–3.5, and aggressive control of risk factors: glycemic and blood pressure control, together with discontinuation of oral contraception. Vaccination prophylaxis due to functional asplenia was recommended. Gynecologic examination and further investigation of the anemia was also recommended. Although we initially admitted the microcytic hypochromic anemia could be due to iron deficiency related to menorrhagia, normal ferritin and low transferrin did not indicate sideropenia. Moreover, such a low MCV with a normal red blood cell count could indicate thalassemia trait, a relatively common condition in the patient's geographic origin. RDW did not distinguish iron deficiency from minor thalassemia.31
In addition to regular medical assessment, patient education regarding awareness of signs of arterial embolism is crucial, as aortic thrombus can recur in patients regardless of anticoagulation. The first few hours after an embolic event are critical to avoid irreversible damage.
Conflicts of interestThe author has no conflicts of interest to declare.