Percutaneous valve replacement for severe aortic stenosis has been shown to be an alternative treatment option for high surgical risk patients. We describe our first valve-in-valve procedure in a patient with a degenerated aortic bioprosthesis and severe regurgitation.
O tratamento percutâneo da estenose aórtica severa demonstrou ser uma alternativa terapêutica para os doentes de alto risco cirúrgico. Descreve-se o caso do primeiro doente com bioprótese aórtica degenerada e regurgitação severa tratado por via percutânea no nosso centro.
Percutaneous implantation of aortic valve prostheses is a promising technique that has been shown to be effective and safe in patients with severe symptomatic aortic stenosis who have been refused for surgical valve replacement.1–5 However, previously operated patients whose aortic bioprostheses have since degenerated are often refused further valve replacement surgery due to the invariably high surgical risk, and until recently have had no possibility of effective treatment. In 2007, Wenaweser et al. described the first percutaneous implantation of an aortic valve prosthesis in a patient with a degenerated surgical bioprosthesis and severe regurgitation, using the CoreValve Revalving system (Medtronic Inc., Minneapolis, MN, US).6 Since then, several other cases have been reported by different centers.7–12 We describe our center's first valve-in-valve procedure in a patient with a degenerated aortic bioprosthesis.
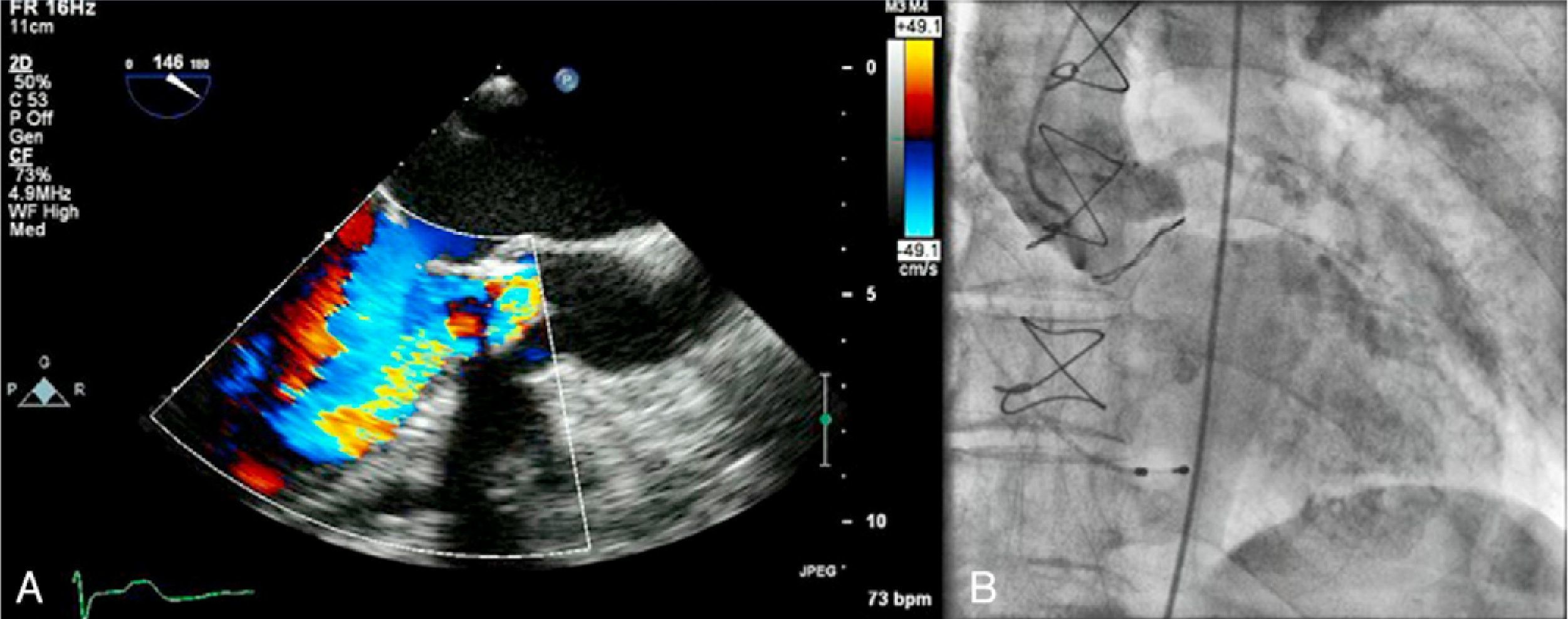
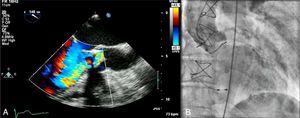
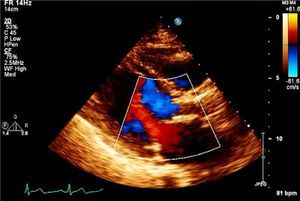
Case reportAn 83-year-old woman, with a history of hypertension (controlled), dyslipidemia, stage III chronic renal failure (creatinine clearance 45ml/min) and chronic anemia (baseline hemoglobin 11g/dl), underwent aortic valve replacement surgery in 2004 with implantation of a 21-mm Mitroflow bioprosthesis (Sorin Group Canada Inc., Burnaby, BC, Canada). She presented with progressively worsening heart failure in New York Heart Association (NYHA) functional class III. The electrocardiogram showed sinus rhythm and no relevant alterations. Transthoracic echocardiography revealed signs of aortic bioprosthesis degeneration, resulting in important valve regurgitation that was difficult to quantify but without significant limitation of valve opening. Other valve structures showed no significant morphological or functional abnormalities and there were no signs of pulmonary hypertension. The left ventricle was of normal size, with preserved global systolic function. Transesophageal echocardiography was performed to characterize prosthetic dysfunction, and showed moderately thickened aortic cuspids and reasonable systolic opening, but central malcoaptation that resulted in two regurgitant jets, one of which was directed anteriorly next to the ventricular septum, moderate to severe (grade III/IV) (Figure 1A). Subsequent cardiac catheterization confirmed significant aortic regurgitation (3+/4+) and excluded significant coronary disease (Figure 1B).
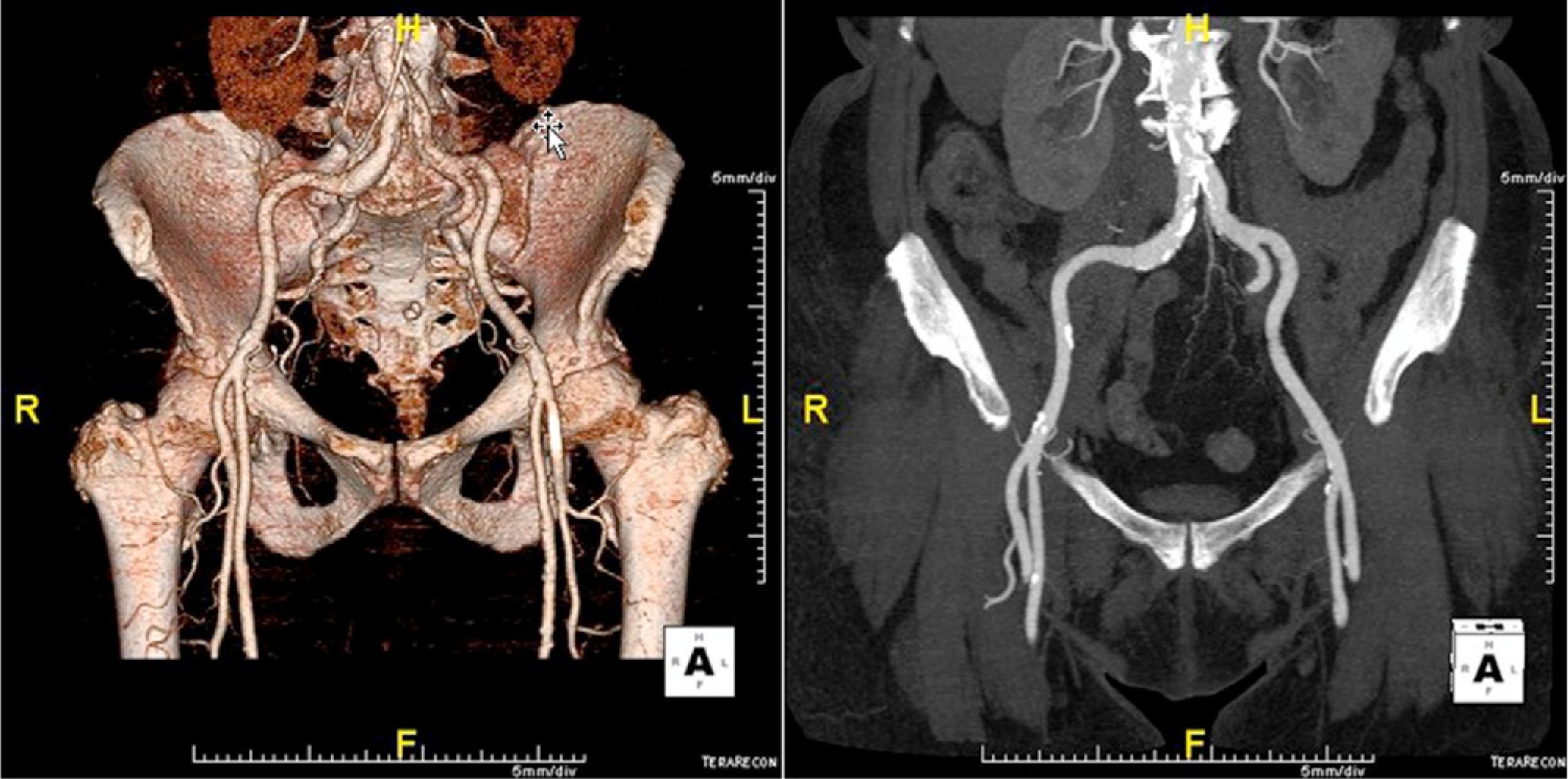
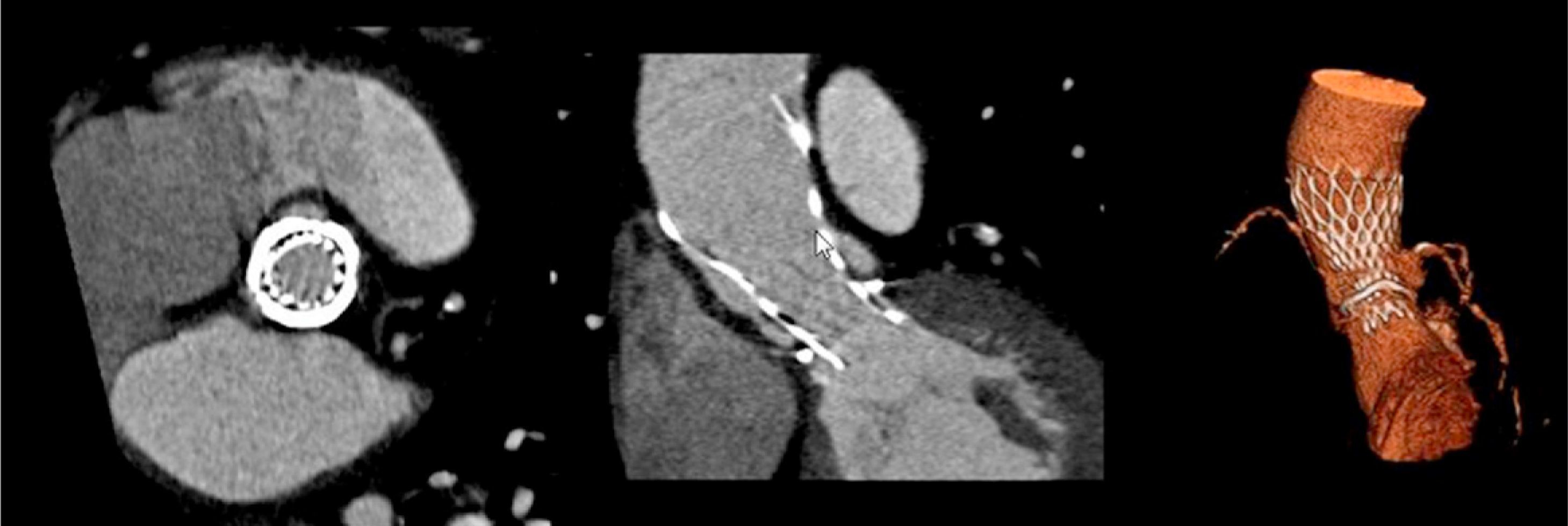
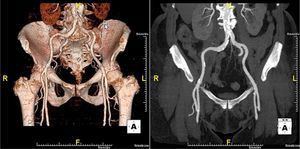
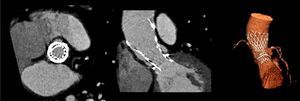
The patient was referred for aortic valve replacement surgery, but was refused on medical and surgical evaluation due to the high surgical risk (logistic EuroSCORE 22.3%). Percutaneous treatment was then considered, and anatomical assessment by transesophageal echocardiography and multidetector computed tomography showed this to be technically feasible. In particular, there was no significant iliofemoral arterial disease that would hinder access (Figure 2).
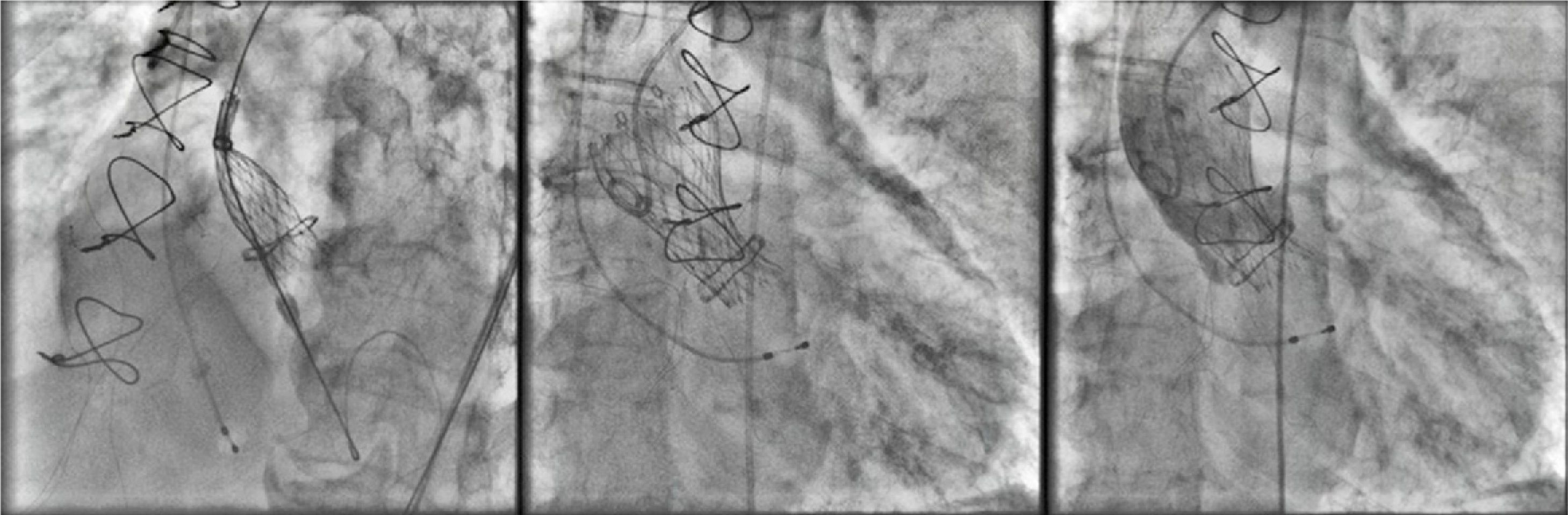
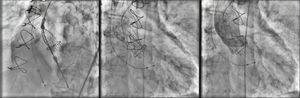
A 26-mm Medtronic CoreValve prosthesis was implanted under general anesthesia via femoral access. The prosthesis was released under rapid pacing for greater control and precision during implantation, the device being positioned higher than is usual for native valves given the smaller diameter of the underlying prosthetic ring. The final angiographic result was excellent, with minimal perivalvular regurgitation (Figure 3).
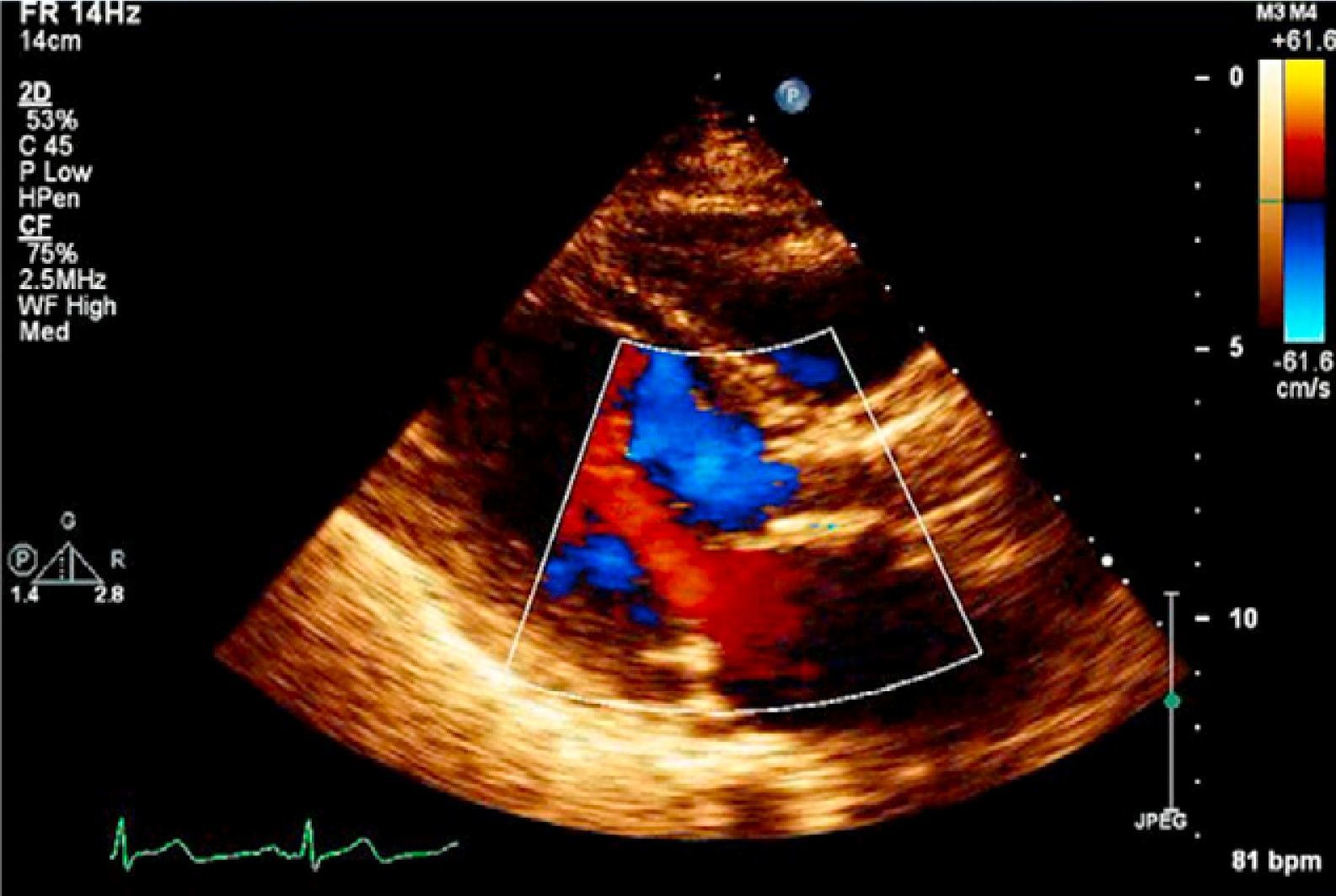
The postprocedural period was uneventful, with no vascular access complications, contrast-induced nephropathy or elevation of myocardial necrosis markers; there were also no arrhythmic complications such as high-degree atrioventricular block (the greater stiffness of the prosthetic ring and implantation in a higher position presumably protected the underlying conduction tissue). Clinically, the patient showed significant improvement in functional capacity and was discharged on the sixth day. The discharge transthoracic echocardiogram confirmed appropriate position and function of the CoreValve and no residual regurgitation (Figure 4). After 14 months of follow-up, the patient remains in NYHA class I and the prosthesis is functioning normally, with no regurgitation (Figure 5).
ConclusionsDespite limited experience to date, the various cases reported by different centers suggest that percutaneous aortic valve implantation is feasible and may be an alternative for patients with degenerated bioprostheses who have been refused for surgery. Nevertheless, the technique remains an off-label indication, which despite its demonstrated feasibility should still be considered a solution of last resort. Further studies are required, with larger numbers of patients and longer follow-up, to determine the role that this technique could play in the treatment of patients with prosthetic dysfunction and high surgical risk.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sousa O, et al. Implantação percutânea de válvula aórtica sobre bioprótese cirúrgica degenerada. Rev Port Cardiol. 2012. doi:10.1016/j.repc.2012.01.014.