We describe the case of a 37-year-old pregnant woman who presented at 29 weeks of gestation with syncope and shortness of breath caused by pulmonary embolism. Due to persistent hypotension thrombolytic therapy with tenecteplase was administered and the clinical and hemodynamic response was excellent, with no maternal or fetal hemorrhagic complications.
The clinical presentation of pulmonary embolism is sometimes camouflaged by the physiological changes that occur in pregnancy and diagnosis is often delayed by reluctance to expose the fetus to ionizing radiation. Systemic thrombolysis is considered a high-risk treatment in pregnancy and very few women have received it. However, the complication rates of thrombolytic therapy are acceptable in the light of the underlying disease.
Grávida de 37 anos de idade com 29 semanas de idade gestacional recorre ao serviço de urgência por síncope e dispneia causadas por tromboembolismo pulmonar com repercussão hemodinâmica. Por hipotensão persistente foi-lhe administrada terapêutica trombolítica com tenecteplase com excelente resposta hemodinâmica e clínica, sem intercorrências hemorrágicas maternas ou fetais.
A apresentação clínica do tromboembolismo pulmonar é por vezes camuflada pelas transformações fisiológicas que ocorrem na gravidez e o diagnóstico é muitas vezes atrasado pela relutância em expor o feto a radiação ionizante. A trombólise é um tratamento de alto risco na grávida e há poucos casos descritos da sua utilização; porém, as taxas de complicações com a terapia trombolítica são aceitáveis em relação à doença subjacente.
It is estimated that 0.2–4% of pregnancies in the Western world are complicated by cardiovascular disease, and this figure is increasing. There is frequently a need for diagnostic and/or therapeutic cardiological intervention in pregnant women, and this is always a challenge, both because most cardiologists lack experience with this patient group and because few cardiological interventions have been thoroughly validated in this population.
The recently published ESC guidelines on the management of cardiovascular diseases during pregnancy were developed more by extrapolating the evidence for non-pregnant patients than on the basis of the limited data available, and most of their recommendations are level of evidence C (consensus of opinion of the experts and/or small studies, retrospective studies, registries).1
Case reportA 37-year-old pregnant woman at 29 weeks of gestation (gravida 2 para 1), with a history of overweight, no relevant family history and not taking any regular medication, presented with fatigue and pain in the side and back of the left thigh 24hours after a short flight of about 3hours. The clinical setting was interpreted as a possible herniated disc with inflammation of the left sciatic nerve, and she was medicated with analgesics and anti-inflammatory agents. Her fatigue worsened, with shortness of breath on successively less exertion; on the ninth day she suffered brief loss of consciousness at home and oppressive chest pain, and went to the emergency department.
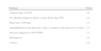
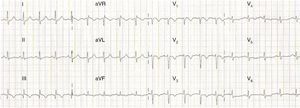
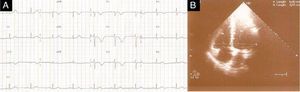
On physical examination she was agitated, hypotensive (73/35mmHg), tachycardic (115bpm), hypoxemic (oxygen saturation in room air 80%) and tachypneic (50cpm). No signs of deep vein thrombosis (DVT) were observed. Laboratory tests revealed hemoglobin 12.3g/dl; platelets 170×109/l; D-dimers 2962ng/ml; and troponin I 0.43ng/ml (reference value <1.50ng/ml). Arterial blood gas analysis (with the patient on 5 l/min supplementary oxygen) showed respiratory alkalosis (pH 7.49); pO2 105mmHg; pCO2 28mmHg, HCO3− 20.9mmol/l; and O2 saturation 98%. The electrocardiogram (ECG) showed sinus tachycardia and signs of right ventricular (RV) overload (Fig. 1), confirmed by bedside transthoracic echocardiography (TTE) (Fig. 2).
Transthoracic echocardiography: (A) end-diastolic apical 4-chamber view showing right ventricular dilatation, paradoxical septal wall motion and pulmonary artery systolic pressure of 65mmHg; (B) parasternal short-axis view showing diastolic flattening of the ventricular septum due to pulmonary hypertension; (C) preserved right ventricular systolic function (tricuspid annular plane systolic excursion 23mm).
The fetus was in transverse position, with good vital signs; the cardiotocogram was reactive, with good variability and no uterine contraction. The ultrasound scan showed concordant fetal growth.
Doppler ultrasound of the lower limbs excluded DVT.
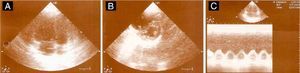
According to the Wells score, the patient's clinical probability of acute pulmonary thromboembolism (PTE) was intermediate (Table 1). She was medicated with subcutaneous enoxaparin 1mg/kg and fluid therapy was initiated. It was decided to perform thoracic computed tomography (CT) with administration of intravenous contrast in the pulmonary arterial phase, the fetus being protected from radiation by lead shielding. CT angiography confirmed acute bilateral PTE (Fig. 3).
The Wells scoring system for diagnosis of PTE.
| Findings | Points |
| Clinical signs of DVT | 3.0 |
| No alternate diagnosis likely or more likely than PTE | 3.0 |
| Heart rate >100bpm | 1.5 |
| Immobilization in the previous 3 days or surgery in the previous 4 weeks | 1.5 |
| Previous diagnosis of DVT/PTE | 1.5 |
| Hemoptysis | 1.0 |
| Cancer | 1.0 |
Clinical probability:
0–1, low;
2–6, intermediate;
>7, high.
DVT: deep vein thrombosis; PTE: pulmonary thromboembolism.
(A) Occlusive thrombus in the right pulmonary artery extending to the lobar branches. An occlusive thrombus can be seen on the left in the superior lobar artery extending to the left pulmonary artery. Another thrombus is visible in the inferior lobar artery, not completely occlusive, extending into the segmental branches; (B) right ventricle measuring 57mm and left ventricle measuring 29mm; (C) coronal plane reconstruction with a thick slice (24mm) in maximum intensity projection, showing thrombus in the right pulmonary artery (red arrow).
The patient was transferred to the coronary care unit, where after four hours she was still agitated, hypotensive (80/45mmHg), tachycardic (115bpm) and tachypneic (60cpm). After weighing the hemorrhagic risk against the greater risk of irreversible clinical decompensation, it was decided to administer thrombolytic therapy with tenecteplase (40-mg bolus over 5 minutes). Within three hours clear clinical and hemodynamic improvement was seen, with blood pressure 95/65mmHg, heart rate 100bpm, breathing rate 25cpm, and decreasing need for supplementary oxygen therapy.
She was discharged from the coronary care unit on the fourth day and transferred to the Obstetrics and Gynecology Department. She remained hemodynamically stable throughout her hospital stay, with 96% oxygen saturation in room air. No maternal or fetal hemorrhagic complications occurred.
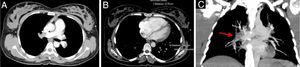
On the fifth day ECG and TTE (Fig. 4) were performed, which showed a normal-sized RV and no signs of pulmonary hypertension. She was discharged home on the 17th day, medicated with subcutaneous enoxaparin 60mg twice a day.
On the 30th day after diagnosis, obstetric ultrasound (Fig. 5) showed fetal growth in the same percentile curve and normal morphology, amniotic fluid volume and flowmetry in the umbilical and middle cerebral arteries. Investigation of maternal thrombophilia revealed her to be heterozygous for factor V Leiden.
DiscussionVenous thromboembolismThe most feared manifestation of venous thromboembolism (VTE) is PTE, a common entity with a mortality of 30% if untreated, mainly due to recurrence. Oral anticoagulation (OAC) at therapeutic doses within 24hours reduces mortality to 2–8%.2,3 In-hospital mortality is 5–17% in patients who present evidence of RV dysfunction at diagnosis4 and 20–30% in those with hemodynamic compromise.5
Venous thromboembolism during pregnancyVTE, which includes DVT and PTE, is the leading cause of maternal death (20%) in developed countries, accounting for 1.2–4.7 deaths per 100000 pregnancies.6 The precise incidence of VTE is unknown but is estimated at 0.5–2 cases per 1000 pregnancies.7 The risk is greatest in the first three weeks after birth by cesarean section,2 but the risk is still high between the third and sixth week after delivery and is the same as during pregnancy. From the sixth week the risk is the same as for non-pregnant women.8
There are three pathophysiological mechanisms, known as Virchow's triad, that together or in isolation may be responsible for the high incidence of VTE in pregnancy9:
- (1)
Venous stasis: This begins in the first trimester and reaches a maximum at 36 weeks. It is caused by progesterone-induced venodilation, compression of the pelvis by the gravid uterus, and pulsatile compression by any of the iliac arteries on the left iliac vein (which explains why 80% of cases of DVT in pregnancy are on the left, a phenomenon known as May-Thurner syndrome)10;
- (2)
Vascular injury: During childbirth the veins of the pelvic region may be distended and/or traumatized, especially when a cesarean section is performed (which explains the greater risk described above);
- (3)
Hypercoagulability: The production of several coagulation factors (I, II, VII, VIII, IX and X) increases in pregnancy, while protein S production and the activity of the inhibitors of fibrinolysis PAI-1 and PAI-2 are reduced. These physiological changes are crucial to the hemodynamic challenges of birth (peripartum bleeding is the leading cause of maternal death in developing countries11). This prothrombotic state will be further exacerbated by the presence of hereditary thrombophilia such as factor V Leiden, the G20210A mutation in the prothrombin gene, antithrombin III or protein C or S deficiency, or the presence of antiphospholipid antibodies.12
The clinical features of VTE can be frustratingly difficult to evaluate, since most healthy pregnant women have lower limb edema and up to 70% suffer from shortness of breath during pregnancy.13 Diagnosis of VTE, and particularly PTE, requires a high index of clinical suspicion, based on predisposing conditions and risk factors (in the case presented, these included overweight, pregnancy at age over 35, thrombophilia, immobility during a flight, and initial symptoms in the left leg compatible with DVT).1,2
Based on risk factors and physical examination, the clinical probability of PTE can be calculated using the Wells or Geneva score. This then guides the choice of diagnostic exams (Table 1).2 These tools have not been validated in the pregnant patient, although one study has found three variables that appear to predict DVT in pregnant women: left leg symptoms, >2cm difference in thigh circumference, and first trimester.14
Laboratory results such as respiratory alkalosis or elevated fibrin degradation products are also commonly found in healthy pregnant women; levels of the latter increase with gestational age and reach a maximum at the time of birth, but such tests should be performed due to their ability to exclude disease and to avoid unnecessary exposure to ionizing radiation.1,15
Imaging studiesPeripheral venous Doppler ultrasoundThe deep venous system of the lower limbs is difficult to assess by physical examination, and when DVT and/or PTE are suspected the techniques used are B-mode echocardiography and compression venous ultrasonography together with color Doppler in transverse view.1,2 Magnetic resonance imaging has 100% sensitivity in diagnosing DVT and appears to be safe in pregnancy.16 Documented DVT in a hemodynamically stable pregnant woman is sufficient motive to begin OAC without needing to exclude or confirm PTE, although at least 70% of patients with PTE do not have DVT at the time of diagnosis.17
RadiographyExams using ionizing radiation in women of childbearing age should be performed during the first 10 days after a menstrual cycle, and if there is a possibility that the woman is pregnant, this must be excluded first. Exposure of the ovaries to radiation pre-conception has no measurable effects on future gestations, and the risk from ionizing radiation to pregnant women is the same as to those who are not pregnant. However, for the fetus, ionizing radiation can cause death, malformations (particular ocular), growth retardation and mutagenic and carcinogenic effects, which depend mainly on gestational age (the most vulnerable period is between the second and eighth week) and the absorbed radiation dose.
A major problem with diagnosis of PTE is clinicians’ reluctance to expose the fetus to ionizing radiation, often due to overestimation of the risk of harm. When faced with the clinical probability of PTE, the primary diagnostic modalities are pulmonary ventilation-perfusion scintigraphy (VPS) and thoracic CT. The estimated radiation dose from CT absorbed by the fetus is 0.003–0.13mGy, while from VPS it is 0.2mGy. There is no evidence that doses of up to 50mGy lead to fetal abnormalities, low IQ, growth restriction or miscarriage. Less radiation is absorbed by the mother's mammary and pulmonary tissue with VPS than with CT.18,19 Although VPS and CT appear to be safe for the fetus, it should be noted that some studies suggest that exposure to low radiation doses in utero can increase the risk of childhood leukemia (1 in 2000 compared to the baseline risk of 1 in 2800), which does not compare with the risk of maternal death from undiagnosed and untreated PTE (15%).20
In a pregnant woman with normal chest X-ray, VPS may be more valuable in diagnosing PTE than CT, since in the latter exam the contrast material can be interrupted by unopacified blood from the inferior vena cava. Conversely, CT should be used when the chest X-ray is abnormal, since it can diagnose other conditions such as pneumonia or other lung disease. Pulmonary angiography should not be used in pregnancy.1,21
Iodinated contrast agents may lead to fetal thyroid dysfunction (although this has never been reported with isolated use), and this should be assessed in the first week after birth.
TreatmentPreventionAll women should be routinely assessed for risk of VTE before conception or in the first weeks of pregnancy. The most important risk factors are history of unprovoked DVT, recurrent VTE, PTE or thrombophilias (for which a family history of VTE is an important factor). Half of the women with VTE during pregnancy have either a thrombophilic disorder or a previous thrombotic event, and there are thought to be identifiable risk factors in around 80% of cases of death from PTE in pregnancy.1,22 Prevention of VTE in pregnancy is without doubt better than cure and prophylactic measures (OAC with enoxaparin 0.5mg/kg and compression stockings) should be taken when the risk is considered to be high or even moderate.1
Acute treatmentOAC, together with unfractionated or low molecular weight heparin, should be administered to achieve therapeutic doses within 24 hours. This reduces mortality by preventing recurrence of PTE and improving RV function. There is no evidence of differences in mortality between OAC alone or in combination with thrombolytics, although in patients with signs of RV dysfunction thrombolysis is associated with less clinical deterioration (10% vs. 25%),23 more rapid resolution of hemodynamic alterations, and probable long-term improvement in pulmonary artery pressure and pulmonary vascular resistance.24 Thrombolysis is indicated in PTE when there is severe clinical instability (i.e. with shock or systolic blood pressure <90mmHg or a fall of >40mmHg in 15 minutes not caused by new-onset arrhythmia, hypovolemia or sepsis), a situation associated with high early mortality (>15%). Although it may be considered in other situations such as severe hypoxemia, severe scintigraphic perfusion defects, RV dysfunction, massive PTE on CT, free-floating thrombus in the right atrium or RV, and patent foramen ovale, there is general agreement on the use of thrombolysis only in the case of persistent hypotension.2 Current thinking is to prescribe thrombolysis not on the basis of the extent or severity of PTE but solely to counteract its hemodynamic repercussions. Pregnancy is a relative contraindication to the use of thrombolytics2, but successful thrombolysis has been reported in at least 200 pregnant women.1 The reported risks are 1% for maternal death, 6% for fetal loss and 8% for hemorrhage, mostly from the genital tract. At the time of delivery, thrombolytic treatment should not be used except in extremely severe cases and if surgical embolectomy is not immediately available. The thrombolytics most commonly used in pregnancy are streptokinase, urokinase and recombinant tissue plasminogen activator (rt-PA). If OAC is absolutely contraindicated, as in the immediate postoperative or postpartum period, possible treatments include an inferior vena cava filter, thrombus fragmentation with or without local thrombolysis, or surgical embolectomy. The use of fluid challenge in PTE-induced hemodynamic compromise is controversial; it should not exceed correction of 500–1000cm3.2
Maintenance treatmentWarfarin should not be used in pregnancy, particularly in the first trimester due to the risk of embryopathy and in the third trimester due to the risk of placental abruption or fetal and neonatal hemorrhage, but can be used after delivery and during breastfeeding. Vaginal delivery is preferable to cesarean section, which should be reserved for specific fetal or maternal indications. It is safe to begin OAC 12hours after delivery; it should be continued for at least three months.1,2
Tenecteplase (TNKase) is a genetically engineered glycoprotein derived from rt-PA by substituting three amino acids, which confers slower plasma clearance, longer half-life, greater fibrin binding, less fibrinogenolysis and coagulopathy, and greater resistance to inactivation by PAI-1. Tenecteplase does not cross the blood-placenta barrier, and single-bolus administration results in more rapid plasmin formation and hence to resolution of the clinical setting.25
A search in PubMed for the keywords “pregnancy” and “tenecteplase” reveals that this is the fifth report of the use of this thrombolytic in pregnancy and the first in the context of PTE (of the previous four cases, two were of myocardial infarction and two of mechanical valve thrombosis).
ConclusionsPulmonary thromboembolism is common in pregnancy and is associated with significant maternal morbidity and mortality. It should always be considered in the presence of suspicious symptoms and signs and confirmed by appropriate diagnostic exams, including VPS or CT. Oral anticoagulation should be begun immediately, and thrombolysis should be considered in cases of hemodynamic instability as it has been shown to be effective in the few cases described in the literature and in the case presented here.
Conflict of interestThe authors have no conflict of interest to declare.