Non-adherence to drug treatment is a major health problem. In Europe, it has been estimated that 9% of cardiovascular events can be attributed to non-adherence. The complexity of dosing regimens is one of the factors identified as contributing to non-adherence. In this systematic review we aimed to assess the impact of dosing frequency on adherence to drug treatment in patients with chronic cardiovascular disease.
MethodsMEDLINE and the Cochrane Library (November 2013) were searched for randomized controlled trials (RCTs) comparing different dosing regimens (once-daily administration vs. two or more daily administrations) and assessing adherence to therapy in patients with chronic cardiovascular disease. Only trials with at least five months of follow-up were included. The results of the studies were pooled through a random effects meta-analysis. Relative risk (RR) and 95% confidence interval (CI) were derived. Statistical heterogeneity was calculated using the I2 test.
ResultsFour RCTs (a total of 2557 patients) were included. Dosing regimens with once-daily administration were associated with a significant 56% reduction in risk of non-adherence to drug therapy (RR: 0.44; 95% CI: 0.35–0.54, I2=25%).
ConclusionsFew clinical trials have assessed the long-term impact of dosing frequency on medication adherence in chronic cardiovascular disease. The best available evidence suggests that taking medication once daily decreases the risk of non-adherence to treatment by approximately 50%. The impact on clinical outcomes remains to be established.
A não-adesão à terapêutica constitui um problema de saúde importante. Na Europa, foi estimado que 9% dos eventos cardiovasculares podem ser atribuídos à não-adesão terapêutica. A complexidade dos esquemas posológicos é um dos fatores apontados como contribuindo para a não-adesão terapêutica. Nesta revisão sistemática pretendemos avaliar o impacto, em doentes com patologia cardiovascular crónica, da frequência posológica na adesão terapêutica.
MétodosPesquisa na MEDLINE e Cochrane Library (novembro 2013) de ensaios clínicos controlados e aleatorizados (RCT) que comparassem, em doentes com patologia cardiovascular crónica, diferentes tipos de regimes posológicos (administração única diária versus duas ou mais administrações) e que avaliassem adesão terapêutica. Foram apenas incluídos ensaios com uma duração de pelo menos cinco meses. Os resultados dos estudos foram agregados através de uma meta-análise (efeitos aleatórios) e calculou-se o risco relativo (RR) e respetivo intervalo de confiança 95% (IC 95%). A heterogeneidade estatística foi calculada com o teste do I2.
ResultadosForam incluídos quatro RCT (2.557 doentes). Os regimes posológicos com administração única diária estão associados a uma redução de 56% do risco de um doente ser não aderente à terapêutica (RR: 0,44; IC 95%: 0,35-0,54; I2=25%).
ConclusõesPoucos ensaios clínicos de longo termo avaliaram o impacto da frequência posológica na adesão terapêutica em doentes com patologia cardiovascular crónica. A melhor evidência disponível sugere que a toma de medicamentos em posologia diária única diminui o risco de não-adesão terapêutica em cerca de 50%. O impacto em termos de outcomes clínicos não está estudado.
hydrochlorothiazide
cardiovascular disease
confidence interval
number needed to treat to benefit
low-density lipoprotein
blood pressure
mean blood pressure
relative risk
Use of a Multidrug Pill in Reducing Cardiovascular Events
Cardiovascular disease (CVD) is the leading cause of death and loss of disability-adjusted life years worldwide.1 Treatment, control and prevention of the consequences of CVD depend on adherence to interventions as much as on those interventions’ efficacy and tolerability. Adherence to treatment includes patients’ behavior in relation to physicians’ recommendations, such as changes in lifestyle, adoption of a specific diet or taking medication.2,3
The World Health Organization recognizes non-adherence to long-term therapies as a major problem that contributes to morbidity and mortality and their associated direct and indirect costs.2–6 The magnitude of non-adherence is estimated at 30–50%,7 for which there are a variety of reasons, including the efforts and strategies used by the physician, the individual characteristics of the patient, and the type, complexity and cost of the therapeutic regimen.8
In this systematic review we aimed to assess the impact of dosing frequency (single vs. two or more daily doses) on adherence to drug treatment in patients with chronic CVD.
MethodsThe electronic databases MEDLINE and the Cochrane Library were searched in November 2013. The search strategy (shown in Supplementary Data Table 1, available online) was adapted from other studies in this area and was extended to searches of references in other systematic reviews and the studies obtained.9
The inclusion criteria were randomized controlled trials comparing different daily dosing regimens (single vs. two or more daily doses) in patients with chronic CVD (coronary disease, hypertension, dyslipidemia or persistent arrhythmia) that provided data on adherence to drug therapy. We arbitrarily set a minimum 5-month follow-up period when selecting trials to assess rates of long-term adherence. Placebo-controlled and double-dummy trials were excluded since they do not allow assessment of the impact of dosing frequency on adherence.
The consistency and interpretability of aggregated results of therapeutic interventions are improved by the ability to disregard the adverse events caused by these interventions.10 In the light of this, the primary outcome selected was non-adherence to therapy rather than adherence. Non-adherence was defined as taking less than 80–90% of the prescribed medication,11 this definition being assumed for studies in which non-adherence was not defined. Data on discontinuation of therapy were not considered to be equivalent to non-adherence, as there can be various reasons for leaving a trial that are related to the drug therapy but not necessarily to the complexity of the dosing regimen, such as tolerability.
Potentially eligible trials were selected independently by two of the authors (DC and JC) after assessment of the abstract and then the complete text. For each of the eligible studies, a standard data collection form was used to enter the population characteristics, interventions and relevant outcomes. Any disagreement between the investigators was resolved by consensus. The possibility of methodological bias in the selected studies was assessed with the aid of the Cochrane Collaboration's tool for assessing risk of bias.12
The results of the individual trials were aggregated by means of a meta-analysis using RevMan software (version 5.2.6; The Nordic Cochrane Centre, The Cochrane Collaboration) to determine the impact of dosing frequency on risk of non-adherence to therapy. Estimates of risk for the combined results and for those of individual studies were assessed using relative risk (RR) rather than absolute risk, since estimates of RR are more consistent between studies with different designs, populations and length of follow-up.13,14 All the study variables were presented with 95% confidence intervals (CI) for the estimated RR. The overall estimate of the magnitude of the effect was calculated using the inverse variance method. When the estimated risk was significant, an absolute measure of sampling effort, the number needed to treat to benefit (NNTB), was estimated.15
The statistical heterogeneity of the results of the different studies was assessed using the I2 test,16 which calculates the percentage of total variation across studies that is due to heterogeneity rather than chance. When there was significant heterogeneity between studies (I2≥50%),17 we considered whether this could at least partially be due to differences in clinical characteristics (type of intervention, underlying disease, duration of study) or in methodology (quality of studies, study design, type of control). The overall magnitude of the effect was estimated by the DerSimonian and Laird method (random effects approach),18 whether or not there was heterogeneity. The random effects model assumes that the results of each study are independent of each other, since each study estimates a different treatment effect. This approach is more conservative than the fixed effects model, which assumes that the effect (magnitude and/or direction) of an intervention is the same in different studies and thus that the differences observed between studies are due to chance.
The risk of publication bias was assessed using Egger's test.19
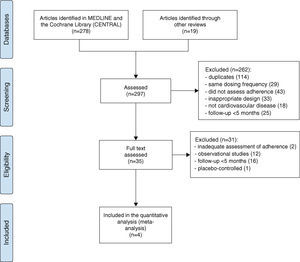
ResultsOn the basis of the inclusion criteria, four clinical trials were selected for analysis.20–23 The results of the assessment and selection process are shown in Figure 1. These trials analyzed 2557 patients with chronic CVD, including patients with hypertension and/or dyslipidemia and high cardiovascular risk. Sample size ranged between 133 and 1921 patients, and follow-up between five and 12 months. In one study the intervention was the polypill (vs. usual care),23 and in the others it was antihypertensive medication.
Based on the aim of the review and the inclusion criteria, the main source of methodological bias in all the selected studies was the fact that they were open; the UMPIRE trial23 was the only one in which investigators were blinded to the treatment results, and even here adherence and non-adherence were reported by the patients themselves, which introduces a different type of risk of bias. A qualitative evaluation of the studies is shown in Supplementary Data Figure 1, available online.
The main characteristics and results of the selected studies are summarized in Table 1.
Main characteristics and results of the selected studies.
| Trial | Population | Interventions and control | Follow-up (months) | Definition of non-adherence | Results |
| Lee et al.20 | 313 hypertensive patients with mild renal dysfunction | Antihypertensive once daily (target BP <92 mmHg) vs. antihypertensive twice daily (target MAP 102–107 mmHg) | 5 | Taking <80% of prescribed pills according to pill count and electronic monitoring | 50% of adherent patients in both intervention and control groups, but only 14% of non-adherent patients achieved target BP |
| Leenen et al.21 | 190 patients with mild hypertension | Amlodipine (once daily) vs. slow-release diltiazem (twice daily) | 5 | Taking <80% of prescribed pills according to electronic monitoring | Non-adherence had a negative impact on BP control in the diltiazem group but not in the amlodipine group |
| Andrejak et al.22 | 133 hypertensive patients | Trandolapril (once daily) vs. captopril (twice daily) | 6 | Taking <90% of prescribed pills according to electronic monitoring | No difference in proportion of patients requiring addition of a diuretic and with controlled BP |
| UMPIRE23 | 1921 patients with high cardiovascular risk or documented CVD | Fixed-dose combination of aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg and 50 mg atenolol or 12.5 mg HCTZ (polypill) (once daily) vs. these drugs taken individually | 12 | Not taking the medication (antiplatelet, statin, and ≥2 antihypertensives) for at least 4 days during the week preceding the end-of-study assessment | Significant reductions in systolic BP (−2.6 mmHg) and LDL cholesterol (−4.2 mg/dl) in the intervention (polypill) group |
BP: blood pressure; CVD: cardiovascular disease; HCTZ: hydrochlorothiazide; LDL: low-density lipoprotein; MAP: mean arterial pressure.
Dosing regimens in which patients with chronic CVD take their drugs once daily were associated with a significant reduction (57%) in risk for non-adherence (RR: 0.44; 95% CI: 0.35–0.54). There was no significant heterogeneity between study results (I2=25%). Figure 2 shows the results of the meta-analysis.
In absolute terms, this reduction translates into a difference of 19% in the proportion of non-adherent patients (95% CI: 12–26%; I2=50%). We also calculated the NNTB that would result in one less non-adherent patient, adjusted to the baseline risk of non-adherent patients prescribed a single daily dose and using the previously obtained RRs. This gave an NNTB of 5 (95% CI: 4–6) over a period of nine months.
Although there was no significant heterogeneity between study results, data in the largest study (UMPIRE23), hence with the most weight in the analysis, were obtained by a self-administered questionnaire and are thus subject to various types of information bias.24.25 In addition, different methods of estimating non-adherence (questionnaires and electronic monitoring) do not always produce a high degree of concordance.26,27 We accordingly performed a sensitivity analysis excluding the UMPIRE trial from the analysis in order to evaluate the consistency of the data. The result was similar (RR for non-adherence: 0.50; 95% CI: 0.38–0.67), without statistical heterogeneity (I2=0%).
The result of Egger's test did not suggest publication bias (p=0.335), although the small number of studies means this possibility cannot be excluded.
DiscussionIn this systematic review and meta-analysis we found that once-daily administration vs. two or more daily administrations is associated with a reduction of about 50% in risk of non-adherence to treatment. Although this subject has been extensively studied in other contexts such as psychiatric disease and HIV infection,28,29 there are few long-term clinical trials assessing the impact of dosing frequency on medication adherence in chronic CVD.
On the basis of the four randomized trials selected, we found that less frequent dosing is associated with a significant reduction in non-adherence to treatment, as found in other areas. However, on the basis of the available evidence, it is still difficult to estimate the precise clinical impact in CVD of the better adherence to therapy seen with less frequent dosing.
A recent systematic review and meta-analysis of epidemiological studies estimated that 9% of all cardiovascular events in Europe could be attributed to poor adherence to vascular medications alone. The results of the largest study included here (UMPIRE), probably the only one with the statistical power to reveal differences in clinical outcomes between groups, showed better control of hypertension of hypercholesterolemia in patients prescribed the polypill.30
In 2010 a study was performed in Portugal specifically on adherence to therapy.31 Of the 561 patients with chronic conditions analyzed, a third had CVD. Patients’ responses to the questionnaires showed that the main reasons for non-adherence related to the drugs themselves were adverse effects and symptomatic improvement followed by discontinuation. The need to take many different medications and/or the complexity of the therapeutic regimen was the main reason for non-adherence in 8.7% of patients. Even for those who did not indicate complexity of the therapy as the main reason for non-adherence, it was considered an important factor affecting adherence by over 40%. Complexity of the therapeutic regimen is thus a significant risk factor for non-adherence to treatment in Portuguese patients.
The available evidence does not identify which drug classes are more prone to non-adherence32; this review only included clinical trials on drugs designed to reduce the cardiovascular risk associated with hypertension and dyslipidemia (although the UMPIRE trial included antiplatelet use, the outcomes were concerned with changes in serum lipids and BP profile).23 A large number of recent trials have studied antithrombotic and antiarrhythmic drugs, but most of them had double-blind and/or double-dummy designs and many were placebo-controlled, and hence could not assess the impact of dosing frequency on medication adherence, since all study arms used the same dosing frequency, including for placebo. The few open-label studies that we identified did not report data on adherence to therapy.
Due to the aims and scope of this review, we did not include clinical trials with short follow-up or observational studies, since the inclusion of such heterogeneous material without unifying factors would have raised various methodological issues and hindered interpretation of the results. However, Coleman et al. recently published a systematic review that included 29 studies (68% short-term clinical trials, some of them placebo-controlled, and 32% observational studies) of chronic CVD and assessed the impact of dosing frequency on medication adherence.33 These authors, analyzing different types of studies from the present review, also concluded that a single daily dose was associated with better adherence to treatment.33
LimitationsThe present study is a systematic review and meta-analysis of clinical trials, not an analysis of data on individual patients. The inclusion in a quantitative assessment (meta-analysis) of studies with different populations (with dyslipidemia, hypertension, and/or high cardiovascular risk), interventions and definitions of adherence could lead to bias and heterogeneity, which would affect the conclusions. However, the low degree of heterogeneity between the results of the studies, and the consistent results of the sensitivity analysis, suggest that the methodology adopted is coherent.
ConclusionsFew clinical trials have assessed the long-term impact of dosing frequency on medication adherence and clinical outcomes in chronic CVD. The best available evidence suggests that taking medication once daily decreases the risk of non-adherence to treatment by approximately 50%.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestD. Caldeira has no conflicts of interest to declare. A. Vaz Carneiro and J. Costa are respectively the Director and Assistant Director of the Center for Evidence-Based Medicine (CEMBE) of the Faculty of Medicine of Lisbon University, which in recent years has provided consulting services in the field of emerging health technologies. None of the firms with whom CEMBE has worked had any direct or indirect input to any stage of this study.
We thank the Portuguese Collaborating Center of the Iberoamerican Cochrane Network.
Please cite this article as: Caldeira D, Vaz-Carneiro A, Costa J. Impacto da frequência posológica na adesão terapêutica em doenças cardiovasculares crónicas: revisão sistemática e meta-análise. Rev Port Cardiol. 2014;33:431–437.