It was with considerable interest that we read the article by Guedes et al. on the HIPOGAIA study,1 which in 2014 studied 479 patients with non-valvular atrial fibrillation (AF) under oral anticoagulation with vitamin K antagonists (VKAs) in primary health care and revealed a moderate degree of pharmacodynamic control using these drugs, with a mean time in therapeutic range (TTR) of 67.4%. A third of patients were inadequately controlled (mean TTR <60%).
The results are interesting, but they need to be put into context in view of changes in prescribing patterns of oral anticoagulants (OACs) in Portugal, particularly of the novel oral anticoagulants (NOACs), also known as non-vitamin K antagonist oral anticoagulants.
NOACs were approved for non-valvular AF prior to the HIPOGAIA study, and three (apixaban, rivaroxaban and dabigatran) have been reimbursed by the state healthcare system for this indication since August 2014, the period covered by the study. Their added therapeutic value, based on convenience and safety,2–4 together with a safety profile at least non-inferior to warfarin, make these drugs an attractive option for patients without contraindications.
Consequently, a substantial proportion of patients have been prescribed NOACs from the start, while others have changed from VKAs to NOACs, particularly those with poor INR control. This means that the population of patients taking VKAs is increasingly a selected one, which may partly explain the results of the HIPOGAIA study, such as the high rate of patients with low TTR in hospital outpatient clinics.5
Evidence that NOACs are now the first-line OACs in Portugal, as recommended in the European Society of Cardiology guidelines on AF,6 is provided by data on prescription of these drugs (independently of therapeutic indication) from INFARMED, the Portuguese Health Authority, and the health consultants IMS Health.
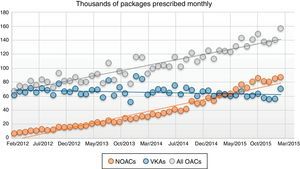
Between 2010 and the first quarter of 2016, the use of NOACs increased, as measured by the number of packages and of defined daily doses (DDDs), a standard measure established by the World Health Organization to assess drug prescribing and dispensing. The peak of VKA use was in 2014, since when it has fallen (Figures 1 and 2).
Besides this change in prescribing patterns, the overall quantity of OACs prescribed has risen significantly (a good sign considering that they had previously under-prescribed7), mainly due to increased use of NOACs. In the first quarter of 2016, the latter already dominated the market in OACs, with 58.4% of packages sold and 51.1% of DDDs (Figure 1).
The era of NOACs has arrived in Portugal.
FundingDC received a grant from Fundação para a Ciência e Tecnologia (FCT) - SFRH/SINTD/96409/2013 - Bolsa Interno-Doutorando.
Conflicts of interestDC and JJF have no conflicts of interest to declare. FJP is a consultant for Astra Zeneca, Bayer and Boehringer Ingelheim.
We thank IMS Health and INFARMED for providing outpatient prescribing and dispensing data on oral anticoagulants.
Please cite this article as: Caldeira D, Ferreira JJ, Pinto FJ. A era dos novos anticoagulantes orais em Portugal. Rev Port Cardiol. 2017;36:577–578.