Anticoagulant therapy is an effective measure in preventing thromboembolic adverse events. Of the diseases in which this treatment is indicated, atrial fibrillation (AF) has the highest incidence worldwide, with a prevalence of 1.5-2%.
ObjectivesTo assess the quality of monitoring of patients with non-valvular AF under oral anticoagulation with vitamin K antagonists in Vila Nova de Gaia healthcare units.
MethodsThis was a retrospective observational analytical study of the population registered at the 37 healthcare units of the Vila Nova de Gaia and Espinho health center area under oral anticoagulation with vitamin K antagonists during 2014. The data were collected using TAONet® software. The variables studied were health units, age, gender, INR value, time in therapeutic range (TTR) and medication. TTR was calculated for each patient using the Rosendaal linear interpolation method. It was stipulated that each patient should have undergone at least six INR measurements. Data were analyzed using Microsoft Excel® 2010 and SPSS® version 21, using descriptive and inferential statistical techniques.
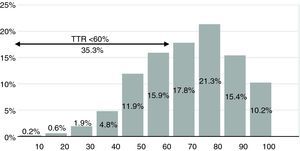
ResultsA total of 479 patients with non-valvular AF were studied, corresponding to 5883 INR tests. Mean TTR was 67.4±6.5%, and 35.3% of patients exhibited poor control (TTR <60%).
DiscussionOur study showed moderate control of coagulation parameters, but better than in many international clinical trials and in another Portuguese observational study. Nevertheless, there is still room for improvement in anticoagulation monitoring in primary health care.
A terapêutica com anticoagulantes é uma medida eficaz na prevenção de eventos tromboembólicos. Das patologias que requerem este tratamento, a fibrilhação auricular (FA) é das que tem maior expressão a nível mundial, com uma prevalência de 1,5-2%.
ObjetivosAferir a qualidade da monitorização de doentes com FA não valvular sob anticoagulantes dicumarínicos, nas unidades funcionais (UF) do concelho de Gaia.
Material e métodosEstudo observacional retrospetivo analítico. População: doentes inscritos nas 37 UF dos ACeS Gaia e Espinho-Gaia sob hipocoagulação com dicumarínicos, durante o ano de 2014. Fonte dos dados: TAOnet®. Variáveis estudadas: ACeS, UF, idade, género, INR, tempo em intervalo terapêutico (TTR) e terapêutica. O TTR foi calculado pelo método de interpolação linear de Rosendaal. Foram consideradas no mínimo seis visitas por doente. Tratamento estatístico: Microsoft Excel® 2010 e SPSS®21.
ResultadosForam estudados 479 doentes com FA não valvular, o que correspondeu a 5883 registos. O TTR médio foi de 67,4% (±6,5). Apresentaram mau controlo da hipocoagulação (TTR<60%) 35,3% dos doentes.
DiscussãoO nosso estudo revela um padrão de controlo de hipocoagulação moderado, mas superior ao encontrado noutros estudos. No entanto, consideramos que ainda há um grande potencial de melhoria nos cuidados de hipocoagulação prestados nos cuidados de saúde primários.
Anticoagulant therapy is an effective measure in preventing thromboembolic adverse events. Of the diseases in which this treatment is indicated, atrial fibrillation (AF) has the highest incidence worldwide, with a prevalence of 1.5-2%.1 According to the FATA study, overall AF prevalence in eight Vila Nova de Gaia family health units (FHUs) was 1.29%.2 AF can cause major hemodynamic changes but prognosis is mainly determined by associated thromboembolic phenomena, which have a significant impact on morbidity and mortality. Stroke, the leading cause of death and disability in Portugal, is five times more common in AF patients.3,4
There are two types of anticoagulant drugs, vitamin K antagonists (VKAs) (warfarin and acenocoumarol) and the new oral anticoagulants (NOACs) (notably dabigatran, rivaroxaban and apixaban). Only the first type require monitoring of international normalized ratio (INR).
VKAs inhibit the production of vitamin K epoxide reductase, thus preventing reconversion to an active form and reducing gamma-carboxylation of glutamic acid residues at sites near the end-terminal of coagulation factors II (prothrombin), VII, IX and X. They also inhibit vitamin-K dependent carboxylation of protein C and its cofactor protein S.5
VKAs have been used for 70 years and were until recently considered the gold standard treatment. They are inexpensive and there is solid evidence that they prevent thromboembolic events in AF patients, one study showing that warfarin reduced ischemic and hemorrhagic stroke by 62% compared to placebo.6 They are also effective in deep vein thrombosis, pulmonary embolism, acute coronary disease requiring stenting, rheumatic valve disease (in the presence of AF or a history of embolism), antiphospholipid syndrome (with history of arterial or venous thrombosis), and valve disease in patients with mechanical or biological prostheses.7 They are also used to prevent thromboembolism following orthopedic surgery.
Nevertheless, there are certain difficulties with the use of VKAs, including a narrow therapeutic window, genetic factors causing interindividual differences in elimination kinetics, and environmental factors such as adherence to therapy, drug interactions and vitamin K dietary intake that can affect their absorption, pharmacokinetics and pharmacodynamics.
Monitoring the effect of these drugs is therefore essential to achieve and maintain adequate levels to prevent thrombotic events while minimizing the risk of bleeding complications. This is done by measuring prothrombin time as expressed by the INR.8,9
There are various ways to monitor oral anticoagulant therapy: (1) in a hospital environment (anticoagulation clinics) by a physician, generally a specialist in hematology or hemotherapy or with experience in the area; (2) in a primary care setting, by a general practitioner (GP), generally the patient's own (routine medical care); (3) in a private laboratory with experience in the area; (4) by the patients themselves using point-of-care devices (self-testing), either self-monitoring, in which patients perform the test at home and then contact their center for dose adjustment, or self-management, in which patients perform the test at home and adjust the dose themselves if necessary.9
Primary or routine care monitoring was first implemented in Portugal 14 years ago, and in 2010, a protocol was established between Centro Hospitalar de Vila Nova de Gaia/Espinho (CHVNGE) and the Espinho-Gaia and Gaia health center groups providing for the monitoring of AF patients with INR within the therapeutic range for at least three months.
Anticoagulation consultations were first decentralized in the Espinho-Gaia group in the Além Douro FHU and progressively extended to 17 units by 2014. Decentralization began in 10 units of the Gaia group in 2013, and remained at this number in 2014.
The guidelines of the Regional Health Authority of the North region stipulate that a coordinator and a team consisting of two GPs and two family practice nurses should be responsible for managing the program, under the supervision of a specialist in hemotherapy from the reference hospital, together with a procedure manual based on principles of good practice. There should also be a computerized database with patients’ history, print-outs of diagnoses, INR values and ranges, drugs prescribed (proposed treatment), next scheduled appointment, and an algorithm to guide prescription and scheduling of appointments, which are subject to validation or change by the physician responsible.
In the Regional Health Authority of the North region, initial anticoagulation monitoring is performed by practice nurses, who determine INR using CoaguChek XS Plus or XS Pro® meters. The patient then sees his or her GP, who adjusts the dosage if necessary and schedules the next visit. The data are entered in the TAONet® system.
At the same time, six-monthly laboratory quality control is performed at the CHVNGE, which can be consulted on a day-to-basis. A fast track system for patients under anticoagulant therapy has also been established.
The quality of a center's anticoagulation control can be assessed by calculating the percentage TTR of the patients monitored there, low values being associated with adverse events. TTR in a given center can be determined in various ways, most commonly by one of three methods: (1) as the fraction of INR values that are within therapeutic range; (2) by analysis of a cross-section of patient records to determine the percentage of patients whose INR is within the therapeutic range at a given point in time compared to the total number of patients with measured INR at that point in time; or (3) by applying the Rosendaal linear interpolation method, which assumes that there is a linear relationship between two consecutive INR values and allocates a specific INR value to each day between tests, thus enabling the number of days within the therapeutic range to be calculated.10
Each approach has its advantages and disadvantages, and various factors can affect the variability of results. Several studies have compared the different methods but were unable to recommend one over another due to methodological issues.11–13 Nevertheless, the NICE guideline of August 2014 recommends the Rosendaal method to monitor anticoagulant therapy in AF patients.14
The aim of this study was to assess the quality of monitoring anticoagulant therapy with VKAs in patients with non-valvular AF in healthcare units of the Espinho-Gaia and Gaia health center groups, using the Rosendaal method.
MethodsThis was a retrospective observational analytical study of patients under VKA therapy registered at healthcare units in the Espinho-Gaia and Gaia health center groups, of which the following 27 out of the total of 37 (73%) provide anticoagulation monitoring: the Primary Health Care Units (PHCUs) of Madalena, Marinha, Crestuma, Lever, Perosinho, Silvalde and Viver Saúde, and the FHUs of Aguda, Além D’Ouro, Anta, Espinho, Caminho Novo, Canelas, Grijó, Monte Murado, Nova Via and São Félix da Marinha in the Espinho-Gaia group; and the following in the Gaia group: the FHUs of Arco do Prado, Gaya, Nova Salus, Saúde no Futuro, Camélias and Abel Salazar, and the PHCUs of Barão do Corvo, Soares dos Reis, Oliveira do Douro and Avintes. In order to protect data confidentiality, the units fulfilling the inclusion criteria were allocated a letter of the alphabet from A to Z in descending order of number of patients.
The variables studied were sociodemographic characteristics, INR values, TTR and current therapy. Patients were considered to be monitored at a healthcare unit if they had a minimum of six visits with INR measurements in 2014.15 INR values were classified as subtherapeutic (<2), therapeutic (2-3) or supratherapeutic (>3). Records were searched for patients with a therapeutic range of 2-3, any with different ranges being excluded.
The Rosendaal method16 allocates a person-time for different levels of anticoagulation assuming a linear relationship between consecutive measurements. The person-time within the target therapeutic range is calculated as a proportion of the total person-time of follow-up. The percentage of days within the target range is expressed as the ratio between the difference between two consecutive INR values in range divided by the total INR difference, as expressed in the formula17:
We opted for this method as it enables comparison with the results of other Portuguese and international studies.15,18–20
Poor control was defined as TTR <60%,6,14,15 moderate control as 60-75%, and good control as >75%.6
All records for 2014 were collected using TAONet® software. The data were recorded and analyzed using Microsoft Excel® 2010 and SPSS® version 21, with no information capable of identifying patients. A descriptive analysis was performed, calculating prevalences and standard deviations. The chi-square test with a 95% confidence interval was used to analyze the association between variables; a value of p<0.05 was considered statistically significant.
Informed consent was considered unnecessary since no data identifying patients were collected or included in the database. Approval for the study was obtained from the ethics committee of the Regional Health Authority of the North region.
ResultsOf the 8249 INR records, 1601 were excluded because either there was no diagnosis of non-valvular AF (359) or there was no diagnostic code (1242); the remaining 6648 records corresponded to 596 patients with non-valvular AF.
In addition, 259 records were excluded as repeats, 76 for showing different values on the same date and 430 of patients with less than six visits recorded. The final number of records was thus 5883. One unit only had patients with less than six visits, and so the number of units analyzed was 26 rather than 27. The records corresponded to 479 patients, of whom 301 (62.8%) were followed in the Espinho-Gaia group and 178 (37.2%) in the Gaia group. Loss to follow-up was 19.6%.
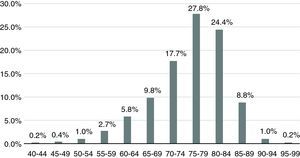
Mean age was 75.6±8.2 years, range 41-96 years. The most prevalent age-group was 75-84 years, accounting for 52.2% of the sample, and 89.8% of patients were aged 65 or over (Figure 1). There was a higher prevalence of women (51.6%).
The most commonly used anticoagulant was warfarin (86% of cases), the other 14% being prescribed acenocoumarol.
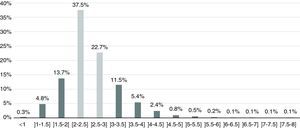
As seen in Figure 2, 60.3% of the sample were within the target therapeutic range (INR 2-3 inclusive), while 39.8% had values outside the range - 18.8% with subtherapeutic INR (<2) and 21% with supratherapeutic INR (>3). High bleeding risk (INR >4.5) was identified in 1.8% of the sample and 5% presented high thrombotic risk (INR <1.5).
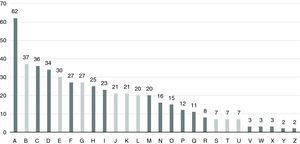
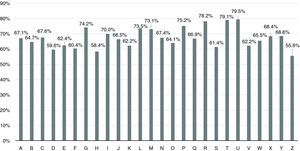
The 26 units were allocated a letter of the alphabet from A to Z in descending order of number of patients (Figure 3). The mean number of patients followed per unit was 18±14 (range 2-62).
The mean number of visits was 12.9±3.2. Unit E showed the lowest mean, with 6.6 visits for 30 patients, while unit W had the highest mean, with 21.7 visits for three patients, as shown in Figure 4.
Figure 5 shows that 35.3% of patients presented poor control, 29.2% moderate control, and 35.5% good control.
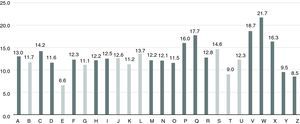
Mean TTR was 67.4±6.5%, with 66.6±6.2% in the Espinho-Gaia group and 68.9±7.2% in the Gaia group, a difference that was statistically significant (p=0.03). As seen in Figure 6, TTR varied between 55.6% and 79.5%, with three units presenting values below 60% and four units achieving rates above 75%.
A finding of poor control did not appear to be related to the number of patients followed by a particular center (p=0.17). No statistically significant association was found between INR control and gender (p=0.35) or with the anticoagulant drug used (p=0.079).
DiscussionThe mean TTR in the Espinho-Gaia and Gaia health center groups was 67.4±6.5%, which indicates moderate control according to the established cut-offs.
Recent large-scale clinical trials comparing NOACs with warfarin in non-valvular AF provided important data on the quality of oral anticoagulant therapy with VKAs, using the Rosendaal method. Mean TTR in our study was higher than reported in international studies. The ROCKET-AF clinical trial comparing rivaroxaban with warfarin, which included patients from 45 countries, found a mean TTR at the individual patient level of 55.2±21.3%. The data for Western Europe (16% of the sample) showed a mean TTR of 66.6±17.7%.18 In the ARISTOTLE trial comparing apixaban with warfarin, median TTR was 66%,19 and in the RE-LY trial comparing dabigatran with warfarin, in patients from 44 countries including Portugal,20 mean TTR was 67.2% overall and 61% for the Portuguese population.14 In a study of outpatients attending the anticoagulation clinic of a Portuguese hospital, mean TTR in patients with non-valvular AF was 59.3±19.8%.15
The higher the percentage of TTR, the lower the risk of adverse events. Outcome data from the SPORTIF III and IV trials revealed that 43% of events occurred in AF patients taking warfarin with poor control of TTR (<60%).6 Our study showed poor control in 35.3% of patients, meaning that a significant proportion were at risk. Of these, 10.8% presented INR <1.8, representing a higher risk for ischemic stroke, and 9.5% had INR >3.5, associated with a higher risk of intracranial hemorrhage.18
There was considerable variation in the number of patients followed in each unit, but this was not reflected in statistically significant differences in TTR values. There were a mean of 12 visits over one-year follow-up, about one visit per month, which may be excessive. Although there is disagreement concerning testing frequency, some authors suggest up to 12 weeks for patients with stable INRs without increased bleeding or thromboembolic risk.21
The strong points of our study are an inclusion criterion of a minimum of six INR measurements, since INR tends to vary more at the beginning of therapy,15 defining TTR levels to determine quality of anticoagulation control,6 and the use of Rosendaal's linear interpolation method to calculate TTR at the individual patient level.16 It is also a pioneering study that reflects the situation regarding primary care monitoring of oral anticoagulation with VKAs in the municipality of Vila Nova de Gaia in north Portugal, and is reproducible.
The study also has certain limitations, including information bias from the use of INR records and because 11.5% of records were excluded. A further limitation is that it was unknown whether patients were under initial or chronic anticoagulant therapy, whether invasive procedures may have prompted suspension of therapy, and whether environmental and/or genetic factors influenced the results; the method of dose adjustment (automatic TAONet® protocol or unit protocols) was also unknown. In addition, the overall loss of patients to follow-up (19.6%), the loss of records and lack of diagnostic coding (15.1% of cases) may have given rise to selection bias.
It is worth considering extending the study to other health center groups in the future using the same methodology, in order to assess the situation for the country as a whole. The TAONet® platform should be modified to include mandatory fields and more sociodemographic data. Accurate recording of diagnoses, dosages and visit outcomes should also be encouraged. Further training of health professionals who manage anticoagulant therapy in primary care may be necessary.
In addition, we should stress the importance of adherence to treatment and compliance with dietary recommendations (to avoid day-to-day variations in intake of vitamin K-rich foods) in order to improve control, as well as the need for systematic investigation of signs of supratherapeutic INR levels, such as bleeding gums or ecchymosis.
In conclusion, our study showed moderate control of coagulation parameters, but better than in other studies. Nevertheless, there is still room for improvement in anticoagulation monitoring in primary health care.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed in humans and/or animals for this research.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank Dr. Luísa Sá for revising this article, as well as Mariette Capinha, Sandra Oliveira and Dr. Álvaro Monteiro for their help in clarifying certain points.
Please cite this article as: Guedes M, Rego C. Estudo HIPOGAIA: monitorização da hipocoagulação oral com dicumarínicos no concelho de Gaia. Rev Port Cardiol. 2016;35:459–465.