Acute myocarditis is often misdiagnosed, and its evolution is not always benign; correct and prompt diagnosis is therefore essential.
We report the case of a 62-year-old woman with chest pain after a stressful event and ST-segment elevation on the electrocardiogram, in whom urgent cardiac catheterization showed normal coronary arteries and left ventricular apical ballooning, suggesting takotsubo syndrome. However, cardiac magnetic resonance imaging showed lesions typical of acute myocarditis, thus suggesting this diagnosis.
We highlight the diagnostic difficulty in patients with chest pain, elevated troponin and normal coronary arteries, and the key role of cardiac magnetic resonance in differential diagnosis.
A miocardite aguda é uma entidade frequentemente subdiagnosticada e com uma evolução que nem sempre é benigna, pelo que o seu diagnóstico se torna mandatório.
Apresenta-se o caso de uma mulher de 62 anos, com dor precordial anginosa após evento de stress emocional, associada a elevação do segmento ST no eletrocardiograma, cujo cateterismo cardíaco de urgência mostrou artérias coronárias normais e balonização apical do ventrículo esquerdo, sugestivo da síndroma de takotsubo. Contudo, a ressonância magnética cardíaca mostrou lesões compatíveis com miocardite aguda, revelando, assim, este diagnóstico.
Destacamos a dificuldade diagnóstica no doente com dor torácica, elevação de troponina e artérias coronárias normais, em que a ressonância magnética cardíaca assume um papel fundamental no diagnóstico diferencial.
In patients admitted to the emergency department (ED) with chest pain, distinguishing between ischemic and nonischemic etiology can be a challenge. Differential diagnosis is based on clinical history and diagnostic exams.1
Patients with hemodynamically significant coronary lesions are identified by cardiac catheterization, confirming ischemic etiology. However, a definitive diagnosis in patients with acute coronary syndrome and angiographically normal coronary arteries is more difficult: it could be acute myocarditis, takotsubo cardiomyopathy (TC) or myocardial ischemia with normal coronary arteries, each of which has distinct features on cardiac magnetic resonance imaging (CMRI).2
CMRI is a noninvasive exam that provides a unique morphological characterization, including at tissue level. It identifies, localizes and determines the etiology of myocardial lesions, distinguishing inflammation from ischemia.1
The first use of CMRI in acute myocarditis was described in 1991 by Gagliardi et al., for diagnosing the condition in children.3 CMRI assesses the myocardium through T1- and T2-weighted sequences, on which areas with the extracellular and interstitial edema found in acute myocarditis show up as a hyperintense signal on T2 sequences.4 Furthermore, using a paramagnetic contrast agent, irreversible myocardial lesions can be identified by late enhancement sequences, which distinguishe myocarditis from TC. Biopsy of such lesions may reveal foci of active myocarditis, and so, as well as determining the type of lesion, CMRI can identify the best site for endomyocardial biopsy.5
There has also been considerable research in the use of CMRI in TC. Eitel et al.6 and Koeth et al.7 demonstrated focal edema in T2-weighted sequences in patients with TC in segments showing wall motion abnormalities, but no myocardial lesions on late enhancement studies.
In myocardial ischemia with angiographically normal coronary arteries, CMRI shows a perfusion defect in inversion-recovery gradient-echo or T1-weighted steady-state free precession sequences, with evidence of late enhancement in the same area, transmural or subendocardial, if there is necrosis. The location of this necrosis correlates with wall motion abnormalities, enabling a diagnosis of myocardial infarction (MI) in the absence of coronary lesions.2
Case reportWe report the case of a 62-year-old woman with a first episode of chest pain after a family argument. She had no cardiovascular risk factors but had been diagnosed with age-related macular degeneration. She reported no recent illness or therapy.
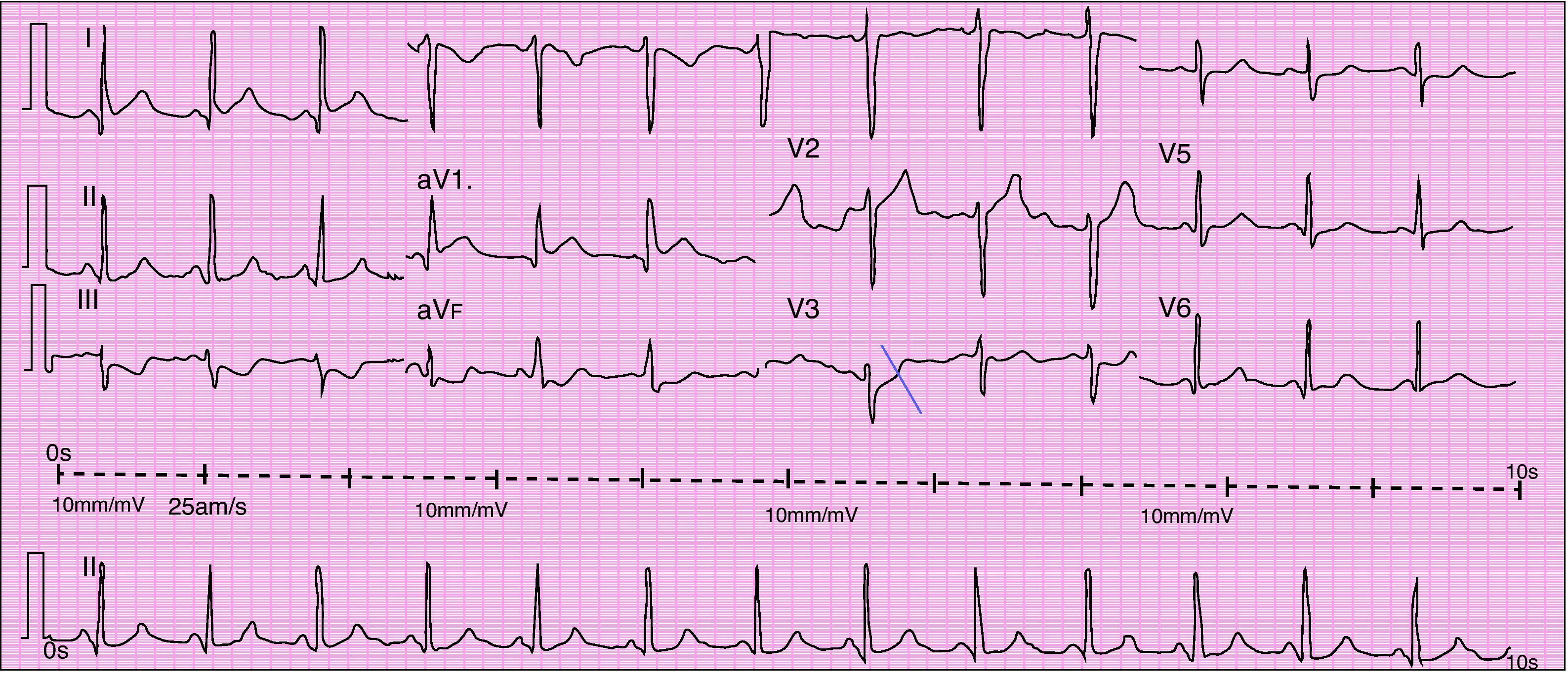
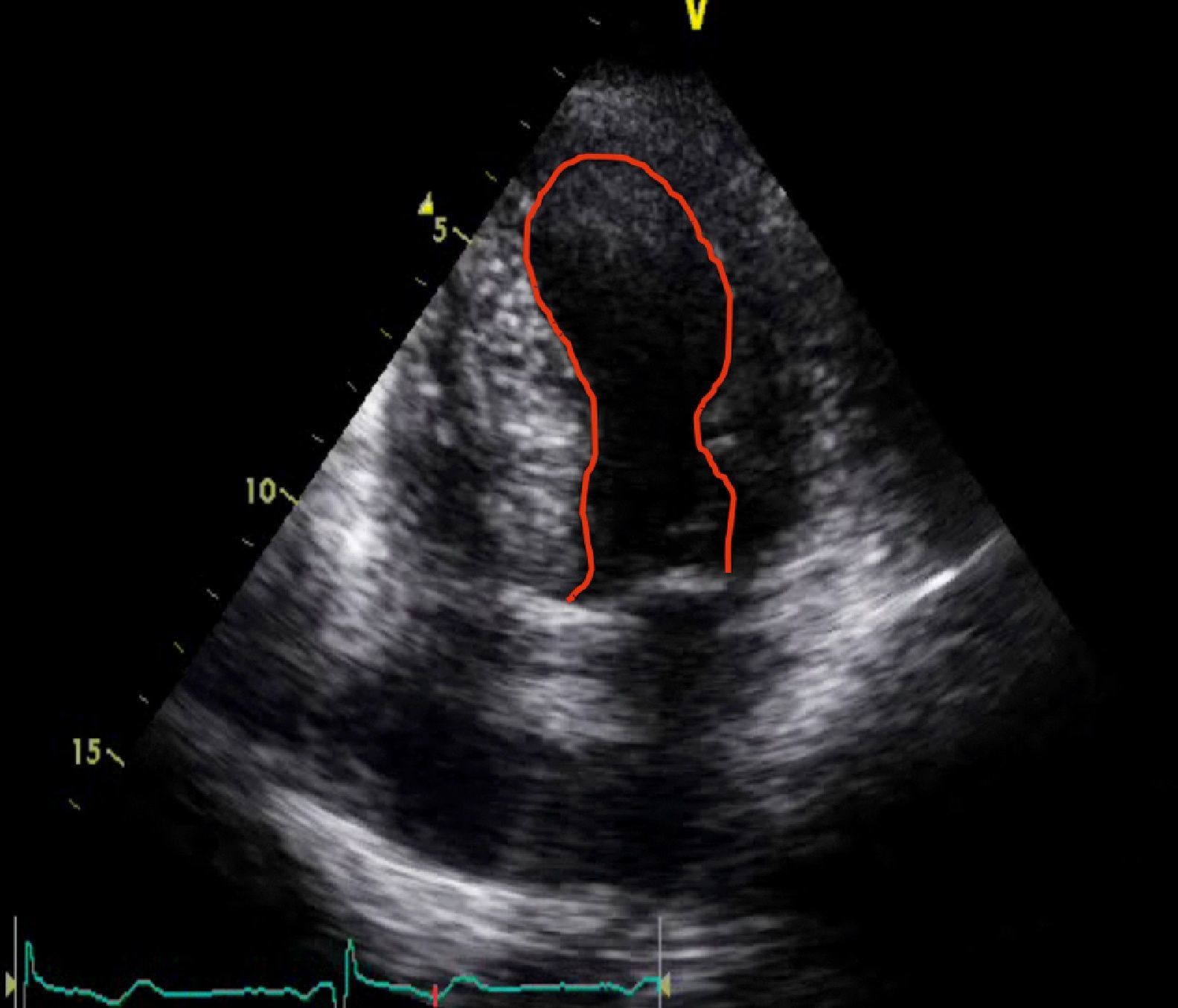
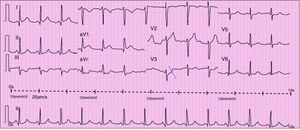
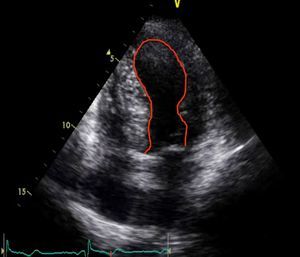
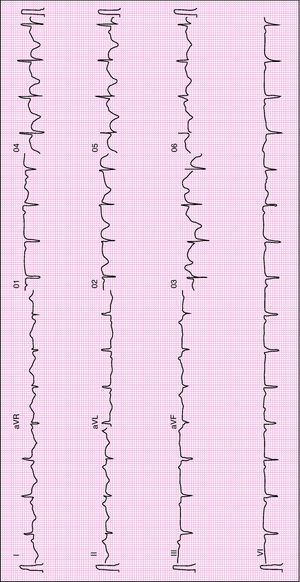
When she went to the ED with chest pain of eight hours’ duration she was hemodynamically stable with no signs of heart failure. The ECG showed sinus rhythm with ST-segment elevation in DI and aVL and ST-segment depression in DIII and aVF (Figure 1). Transthoracic echocardiography performed in the ED showed akinesia with dilatation of the apex and the mid-apical segments of the left ventricular (LV) wall, with no pericardial effusion (Figure 2).
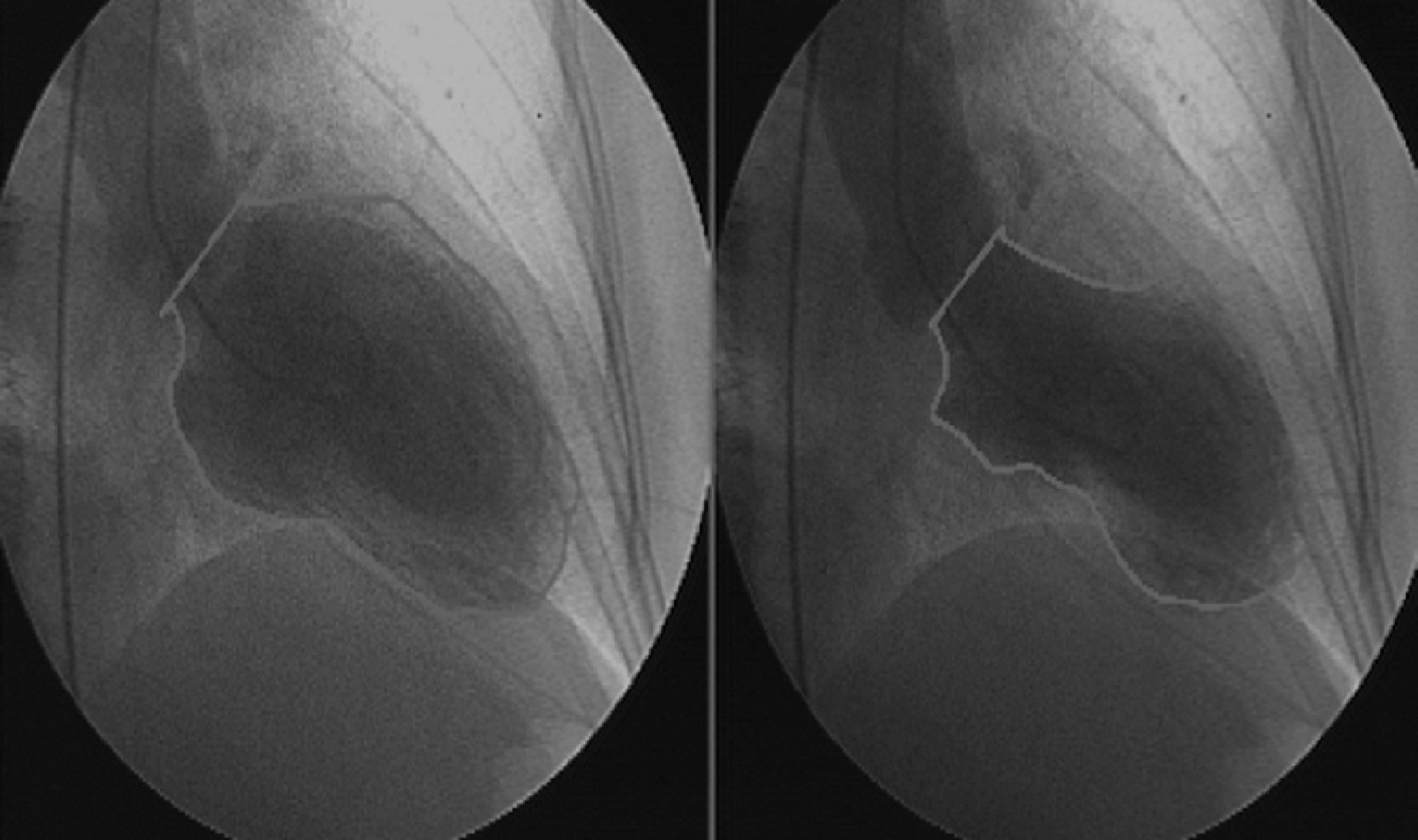
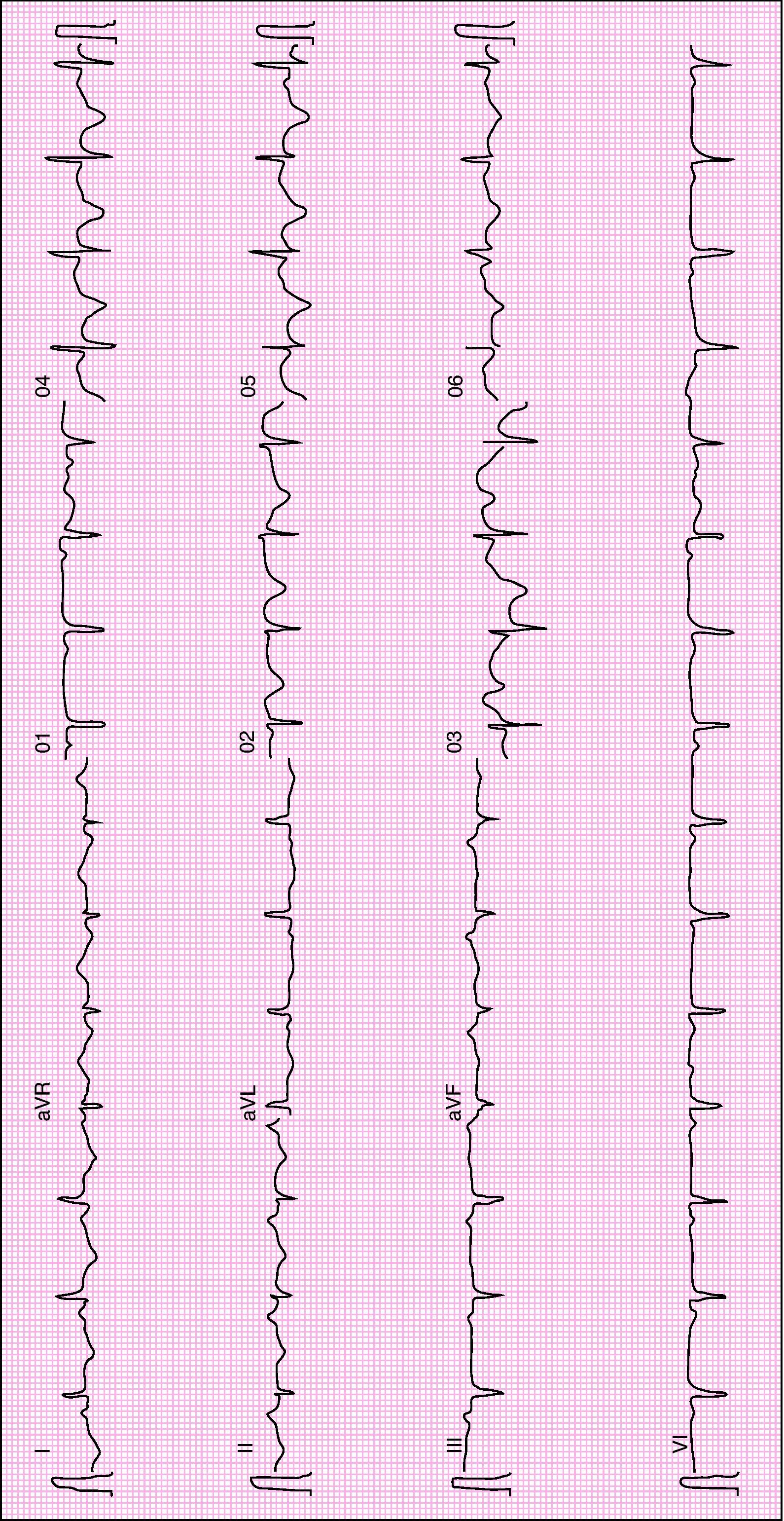
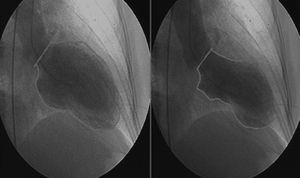
Urgent cardiac catheterization, performed under anticoagulation and dual antiplatelet therapy with aspirin and clopidogrel showed normal coronary arteries and no vasospasm. Ventriculography showed akinesia with apical ballooning and moderate global systolic dysfunction (ejection fraction 42%) (Figure 3). Biomarkers of myocardial necrosis were elevated (peak troponin I 6.45ng/ml and CK-MB mass 27.1μg/dl, with total CK 248μg/dl). C-reactive protein (CRP) was 0.2mg/dl. During hospital stay she was free of chest pain and developed generalized T-wave inversion on the ECG (Figure 4), accompanied by progressive recovery of the LV wall motion abnormalities. Therapy was maintained with dual antiplatelets, anticoagulation, angiotensin-converting enzyme inhibitors (captopril 6.25mg every 8h) and statins (atorvastatin 10mg/day). Beta-blockers were not tolerated due to symptomatic bradycardia.
In view of the clinical setting and results of the diagnostic exams, a provisional diagnosis of TC was made. Since this is a diagnosis of exclusion, 24-h urinary catecholamine assessment was performed, which was normal, as was computed tomography of the adrenal glands. Serology for cardiotropic viruses (adenovirus, Coxsackie, CMV, echovirus, EBV, HZV, HSV-1 and HSV-2, parvovirus B19, influenza, HCV and HIV) in two determinations, despite negative IgM, and autoimmune tests were also negative.
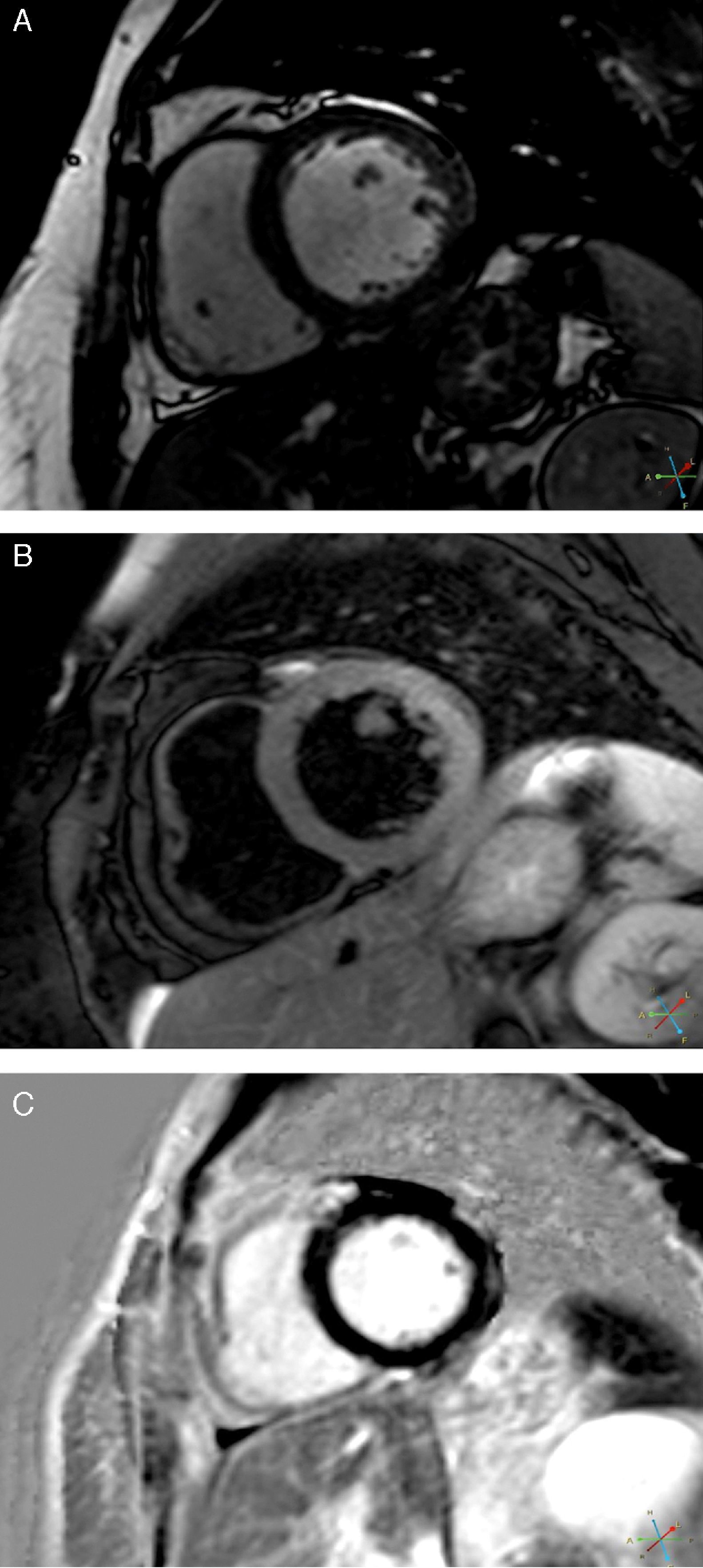
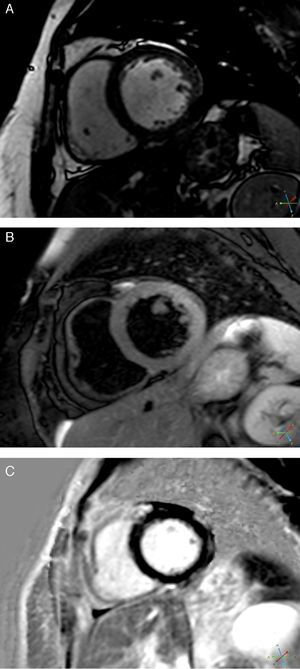
CMRI performed on the 7th day revealed a non-dilated and non-hypertrophied left ventricle, no longer showing wall motion abnormalities and with good global systolic function; there was a hyperintense myocardial signal in T2-weighted turbo spin-echo sequences located in the lateral wall and late gadolinium enhancement in the subepicardium of the same wall (Figure 5). The right ventricle presented no abnormalities.
These findings were compatible with acute myocarditis with involvement of the LV lateral wall, which was in agreement with the ECG changes, although the wall motion abnormalities at admission were compatible with TC.
Treatment with captopril was maintained and ibuprofen 400mg every 8h was initiated. There was complete recovery of wall motion and global LV systolic function and normalization of the ECG pattern, and the patient was discharged on the 10th day with a diagnosis of acute myocarditis.
She has been followed in outpatient consultations and in the following 12 months showed no signs of heart failure or recurrence of angina, with normal transthoracic echocardiogram.
DiscussionTakotsubo cardiomyopathy, also known as transient apical ballooning syndrome, broken heart syndrome and transient left ventricular dysfunction syndrome, is a relatively recently described entity, first reported in 1990 and only recognized as an independent diagnostic entity in 2001, now classified among the stress cardiomyopathies. It accounts for around 2% of cases initially presenting as acute coronary syndrome.8
TC mainly affects postmenopausal women, generally following emotional or physical stress, with a slight elevation of biomarkers of myocardial necrosis. In 2004, the Mayo Clinic published a set of diagnostic criteria for TC, modified in 2007. These are: (1) transient akinesia, hypokinesia or dyskinesia of the LV mid segments with or without apical involvement, the regional wall motion abnormalities extending beyond a single epicardial vascular distribution; (2) absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new electrocardiographic abnormalities (ST-segment elevation or T-wave inversion) or elevation in troponin; (4) absence of pheochromocytoma, myocarditis, hypertrophic cardiomyopathy, intracranial bleeding, or recent significant head trauma. All four criteria must be present.8 Other morphological variants are recognized based on different wall motion abnormalities.8
TC patients typically do not present late enhancement on CMRI, which demonstrates the absence of ischemic myocardial necrosis. Endomyocardial biopsy in the acute phase of TC sometimes shows a slight inflammatory infiltrate and contraction band necrosis that is typical of catecholamine-mediated cardiotoxicity, but these areas are small and often negligible.8 Complete functional recovery is thus relatively rapid, as in the present case.
The case presented is of a postmenopausal woman who, following a stressful event, developed a clinical, electrocardiographic and laboratory setting compatible with ST-elevation MI. However, the LV wall motion abnormalities observed on transthoracic echocardiography indicated the possibility of TC, and this, together with the absence of cardiovascular risk factors, made a diagnosis of MI less likely. Urgent cardiac catheterization excluded coronary artery disease and ventriculography revealed findings typical of TC, making this the main diagnostic possibility.
Other causes of stress cardiomyopathy were excluded, including pheochromocytoma, exogenous catecholamine administration and severe intracranial lesions.8
Since TC is a diagnosis of exclusion, differential diagnosis with myocarditis is essential.
Serology for cardiotropic viruses was performed, even though there were no symptoms suggesting previous viral infection and in the knowledge that its diagnostic accuracy is low; the results were negative, as were autoimmune tests. The next step in the diagnostic process was CMRI, which is now considered the noninvasive exam of choice in the diagnosis of myocarditis.9
The myocardial edema typical of TC,6 associated with evidence of irreversible injury (characteristic of acute myocarditis5) in the same location, was crucial to the diagnosis, since necrosis does not occur, or is negligible and residual, in TC. Subepicardial edema is also a typical finding of acute myocarditis on CMRI. There was a slight rise in biomarkers, as is usual in myocarditis. The underlying etiology could not be identified; viral serology was negative, but a viral etiology is frequently only established by polymerase chain reaction of biopsy specimens.
The final diagnosis in this case was surprising: acute myocarditis presenting with the clinical, echocardiographic and catheterization features characteristic of TC. There is a case report of a patient with both the Mayo Clinic criteria for TC and the Dallas criteria for acute myocarditis on biopsy.10 This highlights the importance of CMRI in patients with an initial probable diagnosis of TC, and of considering whether to perform endomyocardial biopsy.
Endomyocardial biopsy was not performed in the case presented, even though – despite the growing importance of CMRI – it is still considered the gold standard according to the Dallas criteria,11 which define myocarditis on a histopathological rather than clinical basis: the existence of an inflammatory cellular infiltrate with evidence of myocyte necrosis.12 However, endomyocardial biopsy is an invasive procedure and complications are not unknown; besides, its sensitivity and negative predictive value are low, partly due to the characteristically focal nature of myocarditis.5 The latest guidelines therefore recommend it only in patients with rapidly deteriorating cardiac function who fail to respond to conventional medical therapy,13 and it is little used nowadays.
By identifying myocardial edema and lesions on late enhancement images, CMRI led to a noninvasive tissue diagnosis of acute myocarditis. As shown by a study combining CMRI and CMRI-guided endomyocardial biopsy, it is valuable not only in diagnosis but in assessing and monitoring the progression or regression of the disease.5
Despite advances in diagnostic techniques, acute myocarditis is still often underdiagnosed, in part because the initial onset is difficult to recognize clinically.5 Prospective postmortem data have implicated myocarditis in sudden cardiac death of young adults at rates of 8.6–12%, and it has been identified as a cause of dilated cardiomyopathy in 9% of cases,12 and so correct diagnosis is essential.
In the current state of the art, CMRI has a key role in cases of chest pain, elevated troponin and normal coronary arteries, significantly improving diagnostic accuracy.
ConclusionWe report an unusual case of acute myocarditis presenting as TC in a postmenopausal woman with no cardiovascular risk factors.
We highlight the value of CMRI in the differential diagnosis of acute coronary syndromes with normal coronary arteries.
Conflicts of interestThe authors have no conflict of interest to declare.
Please cite this article as: Jorge, C; Síndroma de Takotsubo ou miocardite aguda? O papel da ressonância magnética cardíaca. Rev Port Cardiol 2012. http://dx.doi.org/10.1016/j.repc.2012.06.003.