Novel oral anticoagulants are emerging options for the prevention and treatment of thromboembolic diseases. They are increasingly used in clinical practice due to their simplicity of use and clinical benefits, but an important step is to evaluate their cost-effectiveness. The aim of the AFFORD study (A Review of Cost EFFectiveness of Novel ORal Anticoagulant Drugs) was to perform a systematic review of cost-effectiveness studies of novel oral anticoagulants for stroke prevention in non-valvular atrial fibrillation (AF).
MethodsA systematic review of the literature was conducted by searching the PubMed, Embase, Scopus, Cochrane and Web of Knowledge databases to identify all cost-effectiveness studies of novel oral anticoagulants for stroke prevention in AF.
ResultsThe search identified 27 studies, 18 with dabigatran, three with apixaban, two with rivaroxaban and four with at least two of these drugs. The incremental cost-effectiveness ratios were 30 405±16 101 euros per quality-adjusted life-year (QALY) for dabigatran 110 mg, 17 566±16 902 euros/QALY for dabigatran 150 mg, 8102±3252 euros/QALY for age-adjusted dabigatran, 11 897±3341 euros/QALY for apixaban and 17 960±12 005 euros/QALY for rivaroxaban.
ConclusionThe present systematic review demonstrates that novel oral anticoagulants are cost-effective for stroke prevention in AF.
Os novos anticoagulantes orais são opções emergentes para a prevenção e tratamento das doenças tromboembólicas. São cada vez mais usados na prática clínica pela facilidade do seu uso e pelos seus benefícios clínicos, mas a sua utilização mais generalizada carece de demonstração de custo-efetividade. O objetivo do estudo A Review of Cost EFFectiveness of Novel ORal Anticoagulant Drugs (AFFORD) consistiu na realização de uma revisão sistemática dos estudos de custo-efetividade dos novos anticoagulantes orais na prevenção do acidente vascular cerebral (AVC) na fibrilhação auricular não valvular (FA).
MétodosFoi realizada uma revisão sistemática da literatura nas bases de dados Pubmed, Embase, Scopus, Cochrane e Web of Knowledge para identificar todos os estudos de custo-efetividade dos novos anticoagulantes orais na prevenção do AVC na FA.
ResultadosA pesquisa selecionou 27 estudos, 18 com dabigatrano, três com apixabano, dois com rivaroxabano e quatro com pelo menos dois destes fármacos. Os rácios custo-efetividade incremental por anos de vida ajustados para qualidade foram de 30.405 ± 16.101 euros para o dabigatrano 110 mg, 17.566 ± 16.902 euros para o dabigatrano 150 mg, 8.102 ± 3.252 euros para o dabigatrano ajustado à idade, 11.897 ± 3.341 euros para o apixabano e 17.960 ± 12.005 euros para o rivaroxabano.
ConclusõesA presente revisão sistemática demonstra que os novos anticoagulantes orais são custo-efetivos para a prevenção do AVC na FA.
atrial fibrillation
Canadian dollar
Swiss franc
euro
UK pound
incremental cost-effectiveness ratio
international normalized ratio
oral anticoagulants
quality-adjusted life years
US dollar
vitamin K antagonists
willingness-to-pay threshold
South African rand
Health expenditure is growing faster than wealth creation in most developed countries. In Portugal, per capita state health expenditure rose from 0.3 euros in 1972 to 989.4 euros in 2012, while total expenditure increased from 2.8 million euros (0.2% of gross domestic product) in 1972 to 10 430.5 million euros (6.3%) in 2012.1 State expenditure on drugs, which in 2010 accounted for 17% of total health spending, has risen in parallel with overall health expenditure.1
This investment has led to improvements in health indicators, notably increased life expectancy.2 However, there is growing awareness that the available resources for medical treatments, including drug therapy, are increasingly limited. Economic evaluations are designed to rationalize the use of these resources and to direct them where they are most needed.
In this context, cost-effectiveness analyses are a valuable tool to compare the cost of a health intervention with the expected health gains.3 Interventions include any action intended to improve health that uses financial and/or human resources.
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice,4 and results in a considerable burden in economic terms as well as in morbidity and mortality. Stroke prevention by anticoagulant therapy is the mainstay of AF treatment.5
AF is associated with a prothrombotic state caused by atrial blood stasis and structural heart disease, which predispose to thrombus formation, particularly in the left atrial appendage, and to cardiac embolism. As a result, AF patients have a fivefold greater risk of stroke and systemic embolism than those without AF.5
Anticoagulant therapy is the cornerstone of prevention and treatment of thromboembolic disease.6 Novel oral anticoagulants (OAC) represent a step forward, being easier to use and presenting a more favorable pharmacological profile than vitamin K antagonists (VKA). They also have more rapid onset of action and a more predictable anticoagulant response, eliminating the need for monitoring.6
Phase III clinical trials on four of the novel OAC for stroke prevention in patients with non-valvular AF revealed similar or lower rates of thromboembolism, major bleeding and adverse effects compared to warfarin or aspirin.7–12
Wider use of these new agents could significantly increase the number of adequately anticoagulated patients, since many AF patients do not currently receive any treatment, due to the inconvenience and drawbacks of VKA.4,5
However, without regular monitoring of coagulation levels, larger observational studies are needed to determine the long-term efficacy and safety of the novel OAC in patients with multiple comorbidities and under multiple medication.6 The lack of antidotes, reliable laboratory tests and evidence of safety in real-world clinical practice, together with their high cost, have been identified as important limitations to the wider use of these new agents.
A Review of Cost EFFectiveness of Novel ORal Anticoagulant Drugs (the AFFORD study) is a systematic review of cost-effectiveness studies of novel oral anticoagulants for stroke prevention in AF, and describes their key findings.
MethodsIdentification of studiesStudies that fulfilled the aims of the review were identified using a single search term, “[(adults AND humans) AND (“new oral anticoagulants” OR “new oral anticoagulation” OR “novel oral anticoagulants” OR “novel oral anticoagulation” OR “newer oral anticoagulants” OR “newest oral anticoagulants” OR “new generation oral anticoagulants” OR “oral direct thrombin inhibitor*” OR “new oral thrombin inhibitor*” OR “oral factor Xa inhibitor*” OR “orally active factor Xa inhibitor” OR “orally active thrombin inhibitor” OR rivaroxaban* OR dabigatran* OR apixaban* OR edoxaban*) AND (“cost-effectiveness analysis” OR “cost-effectiveness study” OR “cost-effective” OR “cost-effectiveness”) AND (“atrial fibrillation)]”, in five medical databases: PubMed, Embase, Scopus, Cochrane and Web of Knowledge.
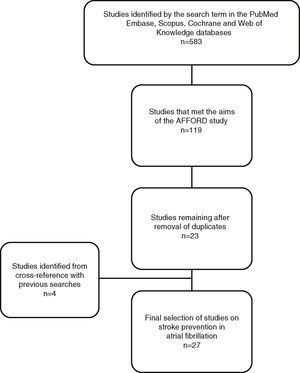
This search identified 533 studies (Figure 1), the abstracts and articles of which were reviewed to select those performed in adult populations comparing new and conventional anticoagulants in terms of cost-effectiveness. Of these, 414 were excluded because they did not meet the required conditions and 52 because they were published as abstracts only.
After elimination of duplicates a total of 23 studies were selected.
A further four studies were selected that did not appear in the results for the above search term; three were in the reference lists of the studies analyzed and one was found in previous searches of PubMed.
Data collectionData related to the pharmacoeconomic model included (1) country; (2) primary comparisons (the new OAC under study, dosages, comparator and daily costs); (3) model structure and assumptions including similarity to ‘progenitor’ models and study perspective; and (4) results including incremental costs, quality-adjusted life years (QALY), incremental cost-effectiveness ratios (ICER), willingness-to-pay thresholds (WTPT) per life-year or QALY, and sensitivity analyses.
Evaluation of quality of studiesThe quality of the studies was evaluated by the investigators on the basis of the inclusion of predefined data on the study models as specified in the criteria of the Quality of Health Economic Studies instrument.13
Statistical analysisThe descriptive nature of this review does not lend itself to formal statistical analysis. The characteristics and results of the pharmacoeconomic models selected were presented qualitatively, supported by figures for incremental costs, QALY, ICER, WTPT and percentages from sensitivity analyses.
Means and standard deviations of ICER in euros per QALY were calculated for each drug after currency conversion when necessary, using the exchange rates on May 16, 2014: 1 US dollar (USD)=0.7321 euros; 1 Canadian dollar (CAD)=0.6736 euros; 1 UK pound=1.2267 euros; 1 Swiss franc (CHF)=0.819 euros; 1 South African rand (ZAR)=0.0708 euros.
ResultsOf the 27 studies selected (Table 1),14–40 most were European (n=11)15,18,19,21,22,24,26–29,33 or American (n=10).14,16,20,31,32,34–36,38,39 Three were Canadian,17,27,37 two Chinese23,30 and one South African.25
Characteristics and results of cost-effectiveness studies of novel oral anticoagulants for stroke prevention in atrial fibrillation.
| Study | Country | New OAC | Price of new OAC | Comparator | Price of comparator | Perspective | Model | Results | WTPT and sensitivity analysis |
|---|---|---|---|---|---|---|---|---|---|
| Freeman et al.14 | USA | Dabigatran 110 mg | USD 8 | Warfarin | USD 1.07 | Health system | Markov | ICER: USD 45372/QALY | 50000 USD/QALYDabigatran cost-effective in 80% of simulations |
| Dabigatran 150 mg | USD 8 | ICER: USD 51229/QALY | Dabigatran cost-effective in 80% of simulations | ||||||
| Kansal et al.15 | UK | Age-adjusted dabigatrana | GBP 2.52 | Warfarin | GBP 0.04 | Health system | Markov | ICER: GBP 4831/QALY – age <80ICER: GBP 7090/QALY – age ≥80 | GBP 20000/QALYProbability of dabigatran being the most cost-effective:age <80: 98%age ≥80: 63% |
| Shah et al.16 | USA | Dabigatran 110 mg | USD 8.88 | Warfarin | USD 0.49 | Health system | Markov | Incremental cost: USD 21300ICER: USD 150000/QALY | USD 50 000/QALYDabigatran 110 mg is not cost-effective |
| Dabigatran 150 mg | USD 8.88 | Incremental cost: USD 20700ICER: USD 86000/QALY | Dabigatran 150 mg is cost-effective | ||||||
| Sorenssen et al.17 | Canada | Age-adjusted dabigatrana | CAD 3.2 | Warfarin | CAD 0.6 | Health system | Markov | Incremental cost: CAD 2178ICER: CAD 10440/QALY | CAD 30000/QALYDabigatran cost-effective in 82% of simulations |
| Dabigatran 110 mg | CAD 3.2 | Incremental cost: CAD 4210ICER: CAD 29994/QALY | Dabigatran cost-effective in 42% of simulations | ||||||
| Dabigatran 150 mg | CAD 3.2 | Incremental cost: CAD 1655ICER: USD 9041/QALY | Dabigatran cost-effective in 81% of simulations | ||||||
| Pink et al.18 | UK | Dabigatran 110 mg | GBP 2.52 | Warfarin | GBP 0.11 | - | Event simulation model | ICER: GBP 43074/QALY | GBP 20000/QALYDabigatran 150 mg dominant vs. 110 mg in 76% of simulations |
| Dabigatran 150 mg | ICER: GBP 23082/QALY | Dabigatran 150 mg dominant vs. warfarin in 94% of simulations | |||||||
| Juanatey et al.19 | Spain | Dabigatran 150 mg | EUR 3.03 | Warfarin | EUR 0.05 | Health system | Markov | Incremental cost: EUR 4851ICER: EUR 17581/QALY | EUR 30000/QALYDabigatran dominant:96.4% of simulations |
| Adcock et al.20 | USA | Dabigatran 150 mg | USD 8 | Warfarin | ND | Societal | Markov | ICER: USD 12286/QALY | USD 50000/QALYDabigatran dominant for daily cost |
| Langkilde et al.21 | Denmark | Age-adjusted dabigatrana | EUR 2.63 | Warfarin | EUR 0.26 | Health system | Markov | Incremental cost: EUR 1866ICER: EUR 6950/QALY | EUR 30000/QALYDabigatran is cost-effective |
| Ali et al.22 | UK | Dabigatran 110 mg and 150 mg | GBP 2.4 | Warfarin | GBP 0.08 | ND | Prospective observational study | Cost of OAC to prevent 1 stroke/year: warfarin GBP 6219; dabigatran 110 mg GBP 28 086.5 and dabigatran 150 mg GBP 25181 | ND |
| You et al.23 | China | Dabigatran 110 mg | USD 8 | Warfarin | USD 1 | Payer | Markov | Incremental cost: USD 16909ICER: dominated by dabigatran 150 mg | U SD 50000/QALYDabigatran cost-effective in 1.6% of simulations |
| Dabigatran 150 mg | USD 8 | Incremental cost: USD 7057ICER: USD 13810/QALY | Dabigatran cost-effective in 50.6% of simulations | ||||||
| Wouters et al.24 | Belgium | Dabigatran 150 mg | EUR 2.68 | Warfarin | EUR 0.32 | Health system | Markov | Incremental cost: EUR 879ICER: EUR 2807/QALY | EUR 20000/QALYDabigatran cost-effective in 99.85% of simulations |
| Bergh et al.25 | South Africa | Age-adjusted dabigatrana | ZAR 24.66 | Warfarin | ZAR 1.2 | Payer | Markov | Incremental cost: ZAR 19037ICER: ZAR 93290/QALY | ND |
| Davidson et al.26 | Sweden | Age-adjusted dabigatrana | EUR 2.82 | Warfarin | EUR 2.12 | Societal | Markov | Incremental cost: EUR 2212ICER: EUR 7742/QALY | EUR 50000/QALYDabigatran is cost-effective |
| Pletscher et al.27 | Switzerland | Dabigatran 110 mg | CHF 4 | Phenprocoumon 2.25 mg | CHF 0.21 | Payer | Markov | ICER: CHF 25108/QALY | CHF 50000/QALYProbability of dabigatran being the most cost-effective: 84% |
| Dabigatran 150 mg | CHF 4 | ICER: CHF 9702/QALY | Probability of dabigatran being the most cost-effective: 95.8% | ||||||
| Age-adjusted dabigatrana | CHF 4 | ICER: CHF 10215/QALY | Probability of dabigatran being the most cost-effective: 97.7% | ||||||
| Andrikopoulos et al.28 | Greece | Dabigatran 110 mg | EUR 2.72 | Warfarin | EUR 0.04 | Payer | Markov | Incremental cost: EUR 4996ICER: EUR 16653/QALY | |
| Dabigatran 150 mg | EUR 2.72 | Incremental cost: EUR 4218ICER: EUR 11400/QA LY | EUR 50000/QALYDabigatran 150 mg cost-effective in 87% of simulations | ||||||
| Miguel et al.29 | Portugal | Age-adjusted dabigatrana | EUR 2.53 | Warfarin | EUR 0.08 | Societal | Markov | Incremental cost: EUR 2978ICER: EUR 8409/QALY | EUR 30000/QALYDabigatran is cost-effective |
| Chang et al.30 | China | Dabigatran | USD 2.3 to USD 2.5 | Warfarin | USD 1.3 | Payer | Markov | ICER: USD 68333/event prevented | ND |
| Dabigatran | USD 1.7 | Warfarin | USD 0.03 to USD 0.04 | ICER: Dabigatran dominant (cost reduction: USD 34350/event prevented) | |||||
| Kamel et al.31 | USA | Dabigatran 150 mg | USD 6.75 | Warfarin | USD 1.04 | ND | Markov | Incremental cost: USD 9000ICER: USD 25000/QALY | USD 50000/QALYDabigatran cost-effective in 87% of simulations |
| Lee et al.32 | USA | Rivaroxaban | USD 6.8 | Warfarin | USD 1.06 | Payer/health system | Markov | Incremental cost: USD 5912IICER: USD 27498/QALY | USD 50000/QALYRivaroxaban cost-effective in 80.1% of simulations |
| Kleintjens et al.33 | Belgium | Rivaroxaban | EUR 2.70 | – | EUR 0.31 | Payer | Markov | Incremental cost: EUR 828ICER: EUR 8809/QALY | EUR 35000/QALYRivaroxaban cost-effective in 87% of simulations |
| Lee et al.34 | USA | Apixaban | USD 6.8 | Aspirin | USD 0.02 | Health system | Markov | Incremental cost: USD 9151IICER: USD 16205/QALY | USD 50000/QALYApixaban cost-effective in 87.5% of simulations |
| Lee et al.35 | USA | Apixaban | USD 6.87 | Warfarin | USD 0.2 | Health system | Markov | Cost reduction: USD 8934 | USD 50000/QALYApixaban cost-effective in80.1% of simulations |
| Kamel et al.36 | USA | Apixaban | USD 7 | Warfarin | ND | Societal | Markov | Incremental cost: USD 3200ICER: USD 11400/QA LY | USD 50000/QALYApixaban cost-effective in 62% of simulations |
| Coyle et al.37 | Canada | Dabigatran 110 mg | ND | Warfarin | ND | Payer | Markov + meta-analysis | Incremental cost: CAD 4184ICER: CAD 66354/QALY | CAD 50000/QALYDabigatran cost-effective in 1.6% of simulations |
| Dabigatran 150 mg | Incremental cost: CAD 2866ICER: CAD 20797/QALY | Dabigatran cost-effective in 50.8% of simulations | |||||||
| Rivaroxaban | Incremental cost: CAD 3396ICER: CAD 55757/QALY | Rivaroxaban cost-effective in 2.1% of simulations | |||||||
| Apixaban | Incremental cost: CAD 3346ICER: CAD 24312/QALY | Apixaban cost-effective in 44.1% of simulations | |||||||
| Harrington et al.38 | USA | Dabigatran 150 mg | USD 7.3 | Warfarin | USD 0.35 | Societal | Markov | Incremental cost: USD 4906ICER: 3190 USD/QALY | USD 50000/QALYDabigatran cost-effective in 40% of simulations |
| Rivaroxaban | USD 7.29 | Incremental cost: USD 925ICER: USD 11150/QALY | Rivaroxaban cost-effective in 14.9% of simulations | ||||||
| Apixaban | USD 10.34 | Incremental cost: USD 7513ICER: USD 15026/QALY | Apixaban cost-effective in 45.1% of simulations | ||||||
| Deitelzweig et al.39 | USA | Dabigatran 150 mg | ND | Warfarin | ND | Payer | Markov | Cost reduction: USD 179 | ND |
| Rivaroxaban 10 mg | Cost reduction: USD 89 | ND | |||||||
| Apixaban 5 mg | Cost reduction: USD 485 | ND | |||||||
| Kansal et al.40 | Canada | Age-adjusted dabigatrana | ND | Warfarin | ND | Payer | Markov | Incremental cost: CAD 1579/patientICER: CAD 6889/QALY | CAD 30000/QALY |
| Rivaroxaban | ND | Incremental cost: CAD 1732/patientICER: CAD 22475/QALY |
The most frequent study perspectives were the health system (n=11),14–17,19,21,24,26,29,33,35 the payer (n=9)23,25–27,30,33,37,39,40 and societal (n=3).20,36,38
A Markov model was used in most studies (93%),14–17,19–21,23–40 while one used a discrete event simulation model18 and one was a prospective observational study.22
Dabigatran was the subject of most of the selected studies (67%).14–31 Apixaban was evaluated in three studies34–36 and rivaroxaban in two.32,33 All the others compared at least two of the new OAC with warfarin.37–40
The most commonly used comparator was adjusted-dose warfarin, and variability in international normalized ratio (INR) control was taken into account in most studies. The only studies not to use warfarin as comparator were Pletscher et al.27 (who used phenprocoumon, the most common VKA in Switzerland, where the study was carried out), and Lee et al.34 (whose study was based on the results of the AVERROES trial10 comparing apixaban with aspirin in AF patients unsuitable for warfarin).
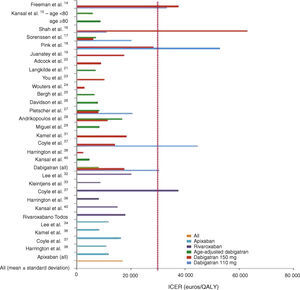
In general, all the studies indicated that the new OAC were cost-effective, with ICERs below the WTPT. The latter was set by the authors but was mainly in agreement with those set by individual national health systems for purposes of reimbursement. Mean ICERs were 30 405±16 101 euros/QALY for dabigatran 110 mg, 17 566±16 902 euros/QALY for dabigatran 150 mg, 8102±3252 euros/QALY for age-adjusted dabigatran, 11 897±3341 euros/QALY for apixaban and 17 960±12 005 euros/QALY for rivaroxaban (Figure 2).
Graphical representation of the results of the AFFORD study, showing incremental cost-effectiveness ratios (ICER) in euros per quality-adjusted life year (QALY) after currency conversion when necessary. The vertical line represents the willingness-to-pay threshold of 30 000 euros per QALY adopted in Portugal.
In studies on dabigatran only, the 150 mg dose tended to be more cost-effective, although there was some variation in sensitivity analyses. Age-adjusted dabigatran (150 mg twice daily for patients aged <80 years and 110 mg twice daily for those aged ≥80 years) was also cost-effective in all the studies in which it was analyzed.15,17,21,25–27,29,40 The 110 mg dose, as well as generally having a higher incremental cost, was not cost-effective in 43% of the models that analyzed it separately.16,23,37
The review also included an economic evaluation carried out in Portugal analyzing the cost-effectiveness of dabigatran for stroke prevention in patients with AF, which included in its analysis both economic data and treatment costs. The clear conclusion was that dabigatran is cost-effective in clinical practice in Portugal.29
The two studies on rivaroxaban, one American32 and the other Belgian,33 both showed that this agent was cost-effective in most simulations in sensitivity analyses.
Of the three studies on apixaban, all carried out in the USA, this agent was associated with savings in a model comparing it with aspirin over 10 years35 and another using warfarin as comparator.35 In the third study, apixaban was cost-effective in 62% of simulations in sensitivity analysis.36
Results of studies comparing all three new OAC37–39 indicate that apixaban is the most cost-effective, followed by dabigatran and rivaroxaban. In Coyle et al.37 and Harrington et al.38 this conclusion is supported by incremental cost and sensitivity analyses, while in Deitelzweig et al.39 medical costs were reduced with the use of all three OAC, the largest reduction being seen with apixaban. Finally, in Kansal et al.’s model of the Canadian setting,40 dabigatran was more cost-effective than rivaroxaban (ICER of CAD 6889/QALY vs. CAD 22 475/QALY, respectively.
DiscussionThe novel OAC have pharmacological advantages over conventional anticoagulants that generally result in clinical benefit, as shown by various trials in a range of clinical settings.7–12
The present review comes at a time when this pharmacological innovation is beginning to be translated into wider use of these new agents in clinical practice.
Since these new drugs are more expensive than VKA, they represent a greater cost burden on health systems and their users. The AFFORD study set out to analyze published economic evaluation studies on the novel OAC and to determine whether they are cost-effective, i.e. whether the health gains exceed the costs of these new drugs. This is the first systematic analysis of cost-effectiveness studies to calculate the mean of the most important variable, ICERs, in euros per QALY (after currency conversion when necessary). These studies, from countries around the globe (North America, Europe, Africa and Asia), differ in their economic models, study perspectives, comparators, drug prices, willingness-to-pay thresholds and presentation of results. This variability in methodology was thus a challenge in comparing the different models.
Nevertheless, the results are consistent, showing that the novel OAC are cost-effective for stroke prevention in AF patients compared to the more widely used conventional anticoagulants, particularly warfarin.
The novel OAC that were shown to be of most interest in this review were dabigatran and apixaban. The former is the subject of more studies, due in part to the fact that it is the oldest of this group. It should be borne in mind that the results of the studies analyzed here are closely related to those of clinical trials. The RE-LY trial on dabigatran in AF showed that the 150 mg dose was more effective and the 110 mg dose was safer than warfarin,7,8 and the 150 mg dose and the age-adjusted dose were also cost-effective in all the studies in which it was analyzed (>80% in sensitivity analysis). Apixaban was superior in both efficacy and safety to warfarin in the ARISTOTLE trial11 and to aspirin in the AVERROES trial.10
It is difficult to compare the results of the models that analyze the three drugs separately, since these are based on studies and trials that use different methodologies, including different study perspectives – payer, health system, or societal. The perspectives of the payer and the health system can be considered equivalent, since the payer perspective can include insurers, employers and the state, which runs the health system in most countries. The societal perspective is wider, since it considers the benefits to the community as a whole; in theory, all costs – both direct and indirect – are included in an economic evaluation from a social perspective.3
This review includes three studies37–39 that analyzed all three novel OAC, and established a hierarchy of pharmacoeconomic performance. Despite differences between the studies, they all point to the same conclusion: the new OAC are cost-effective, and apixaban is the most cost-effective, followed by dabigatran and rivaroxaban. Deitelzweig et al.39 report that all three OAC have a negative incremental cost and therefore produce savings.
Another point in common between most of these studies is the model used for the economic analysis, which was a Markov model in over 90%. This statistical model simulates patients’ clinical course in cycles to the end of their lives; in each cycle a specified probability is applied of the mutually exclusive occurrence of the major events in the population under study.29 Different designs of the Markov model can be used and it can be adapted to different countries and different study perspectives.
The AFFORD study has certain limitations. Indirect comparisons between the novel OAC should be treated with caution due to the different methods used in clinical trials on efficacy and safety. Furthermore, there are no internationally standardized guidelines for conducting economic evaluations, which poses problems for accurate comparisons between different economic models.41 This lack of standardization needs to be remedied, as the increasing concern with containing costs and rationalizing health resource use is leading to a proliferation of economic analyses that must follow generally agreed rules if they are to be comparable.
Another important limitation of the AFFORD study is that some of the authors of the studies included in the review are employed by the laboratories that produce the drugs under study, which could give rise to conflicts of interest.
ConclusionThe AFFORD study demonstrates that novel OAC are cost-effective compared to conventional antithrombotic therapies despite their high cost, in a variety of geographic and social contexts, and when analyzed by different pharmacoeconomic methodologies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ferreira J, Mirco A. Revisão sistemática das análises custo-efetividade dos novos anticoagulantes orais na prevenção do acidente vascular cerebral na fibrilhação auricular: estudo AFFORD. Rev Port Cardiol. 2015;34:179–191.







 ICER) in euros per quality-adjusted life year (
ICER) in euros per quality-adjusted life year (

