Free-floating right atrial thrombi are rare but associated with high mortality. Although advances in echocardiography have improved diagnosis, their management is still the subject of debate. A 24-year-old woman with a history of smoking, obesity and oral contraceptive use presented to the emergency department with dyspnea, cough and hemoptysis. Transthoracic echocardiography revealed a large free-floating cardiac mass occupying the right atrial chamber and restricting tricuspid valve opening. In view of recurrent pulmonary embolism, she was referred for cardiac surgery and the cardiac mass was excised. Anatomopathological analysis revealed an organized and calcified thrombus. Genetic study showed her to be homozygous for the 4G/4G allelic variant of plasminogen activator inhibitor-1 and heterozygous for the allelic variant A1298C of 5,10-methylenetetrahydrofolate reductase.
A presença de trombos móveis na aurícula direita são fenómenos raros, mas associados a uma elevada mortalidade. Apesar de a ecocardiorafia ter permitido avanços no seu diagnóstico a sua abordagem continua a ser motivo de debate. Neste artigo apresentamos o caso de uma doente do sexo feminino, de 24 anos com antecedentes de tabagismo, obesidade e sob terapêutica anovulatória que recorre ao serviço de urgência por cansaço fácil e tosse com expectoração hemoptóica. O ecocardiograma transtorácico revelou massa, móvel, multilobulada de grandes dimensões na aurícula direita, condicionando abertura da válvula tricúspide. Perante episódios recorrentes de embolia pulmonar foi submetida a cirurgia cardíaca com exérese da massa, sendo o resultado anatomopatológico compatível com trombo organizado com calcificação. O estudo genético revelou homozigotia para a variante alélica PAI-1:-675G >A (4G/4G) do inibidor do activador do plasminogénio e heterozigotia para a variante alélica MTHFR 1298 A/C da 5,10-metilenotetrahidrofolato redutase.
Free-floating thrombi in the right cardiac chambers are rare, being found almost exclusively in patients with suspected or confirmed pulmonary thromboembolism.1 Associated mortality is higher than in isolated pulmonary thromboembolism and can exceed 40%, since they are normally an indication of imminent and potentially fatal pulmonary embolism.2,3
Although the latest European Society of Cardiology guidelines on the diagnosis and management of acute pulmonary embolism recommend transthoracic echocardiography in particular circumstances, its routine use could improve diagnosis of right heart thrombi in these patients.2,4
Despite advances in diagnosis, treatment of right heart thrombi is still the subject of debate.1,2
Case reportA 24-year-old woman, morbidly obese (body mass index 40kg/m2), a smoker (1pack/year since the age of 14) and taking oral contraceptives since the age of 17, was apparently asymptomatic until one month before hospitalization, when she began to suffer progressively worsening fatigue on minimal exertion, together with dyspnea and cough with expectoration, mainly mucous but at times bloody. Physical examination revealed tachypnea (24cycles/min), tachycardia (105bpm) and blood pressure of 145/85mmHg. No other significant alterations were observed.
Laboratory tests showed no anemia or elevation of inflammatory parameters or markers of myocardial necrosis, but D-dimers were 3.42μg/ml (reference value: <0.5μg/ml).
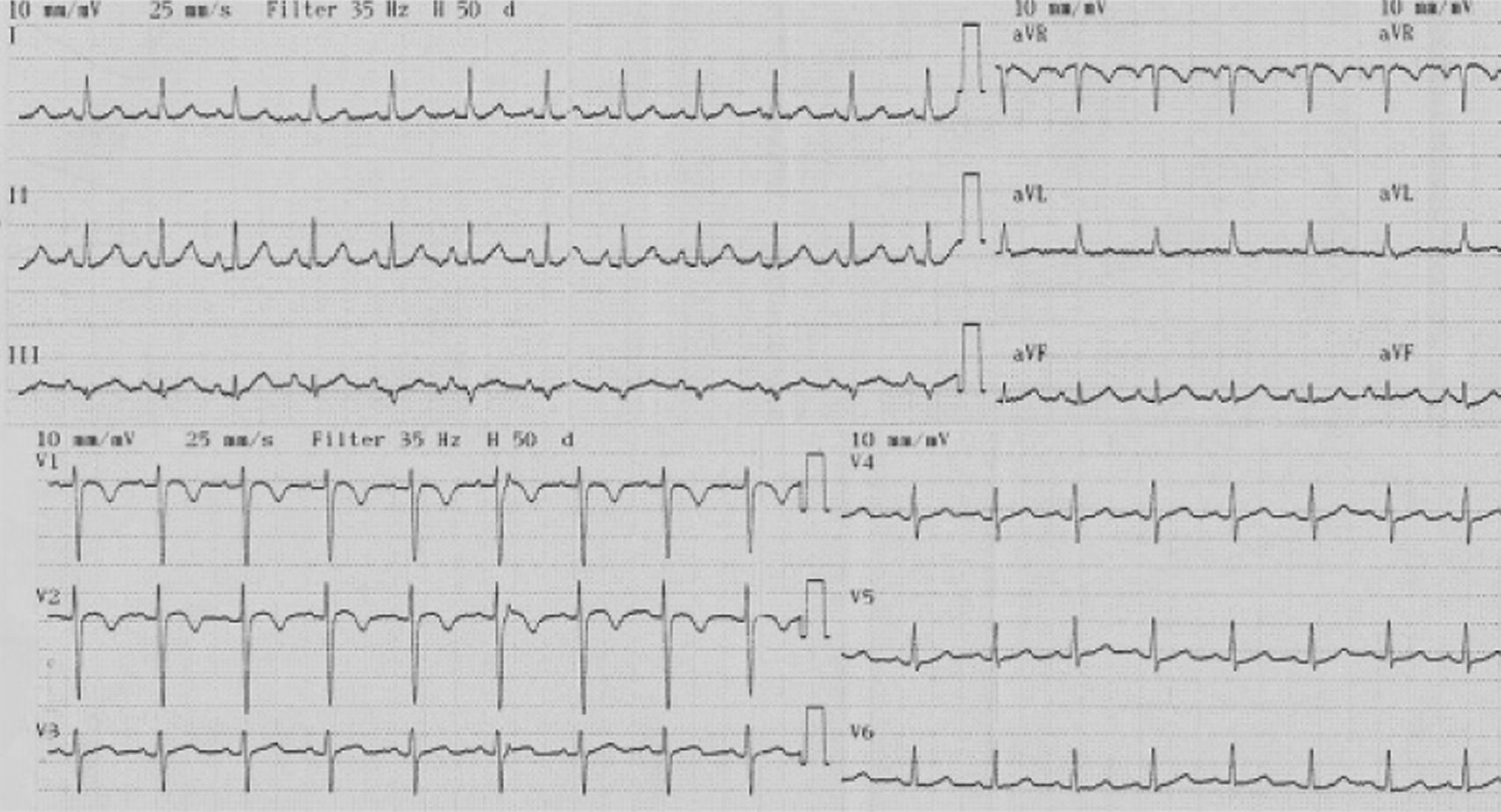
The electrocardiogram (ECG) (Figure 1) showed sinus tachycardia at 110bpm, tall R waves in V1–V3 and T-wave inversion in V1–V2.
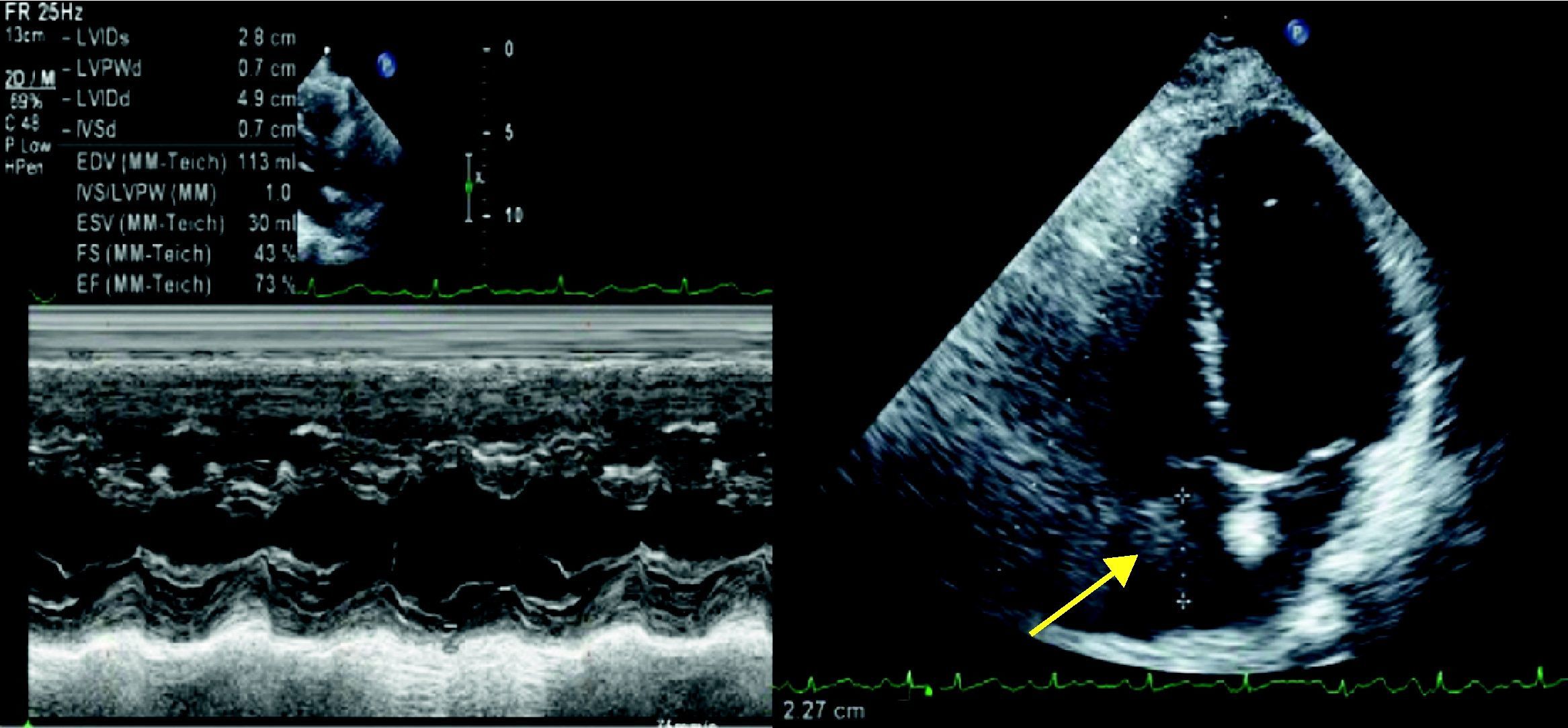
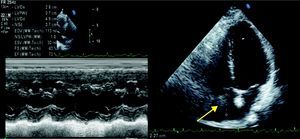
Given the diagnostic hypothesis of pulmonary thromboembolism, and in order to clarify the clinical situation, transthoracic echocardiography was performed, which revealed a large multilobulated mass in the right atrium, protruding through the tricuspid valve into the right ventricle during diastole; absence of left ventricular dilatation or wall thickening; good global and segmental systolic function; and no other significant abnormalities (Figure 2).
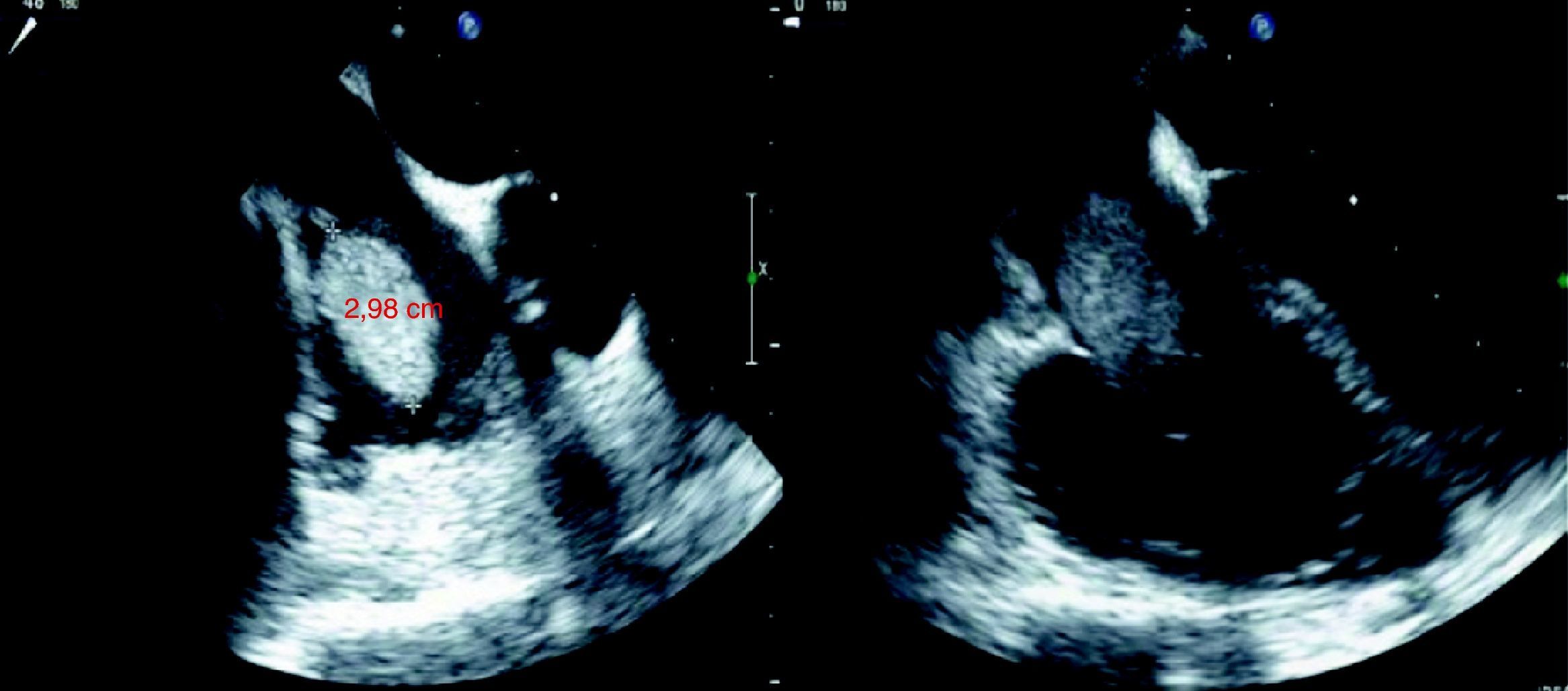
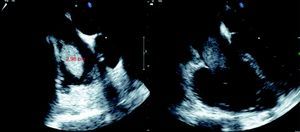
Transesophageal echocardiography confirmed the presence of a multilobulated mass apparently adhering to the roof of the right atrium and protruding through the tricuspid valve (Figure 3).
The patient was immediately admitted and her clinical condition progressively worsened, with recurrent episodes of sudden dyspnea accompanied by bloody expectoration. In view of the diagnostic hypothesis of recurrent pulmonary embolism she underwent urgent right atriotomy and a multilobulated, highly mobile mass, adhering to the Eustachian valve and occupying the entire right atrium, was excised. There were no complications and the patient was discharged under anticoagulant therapy with warfarin.
Anatomopathological analysis of the mass revealed an organized right atrial thrombus with some calcification.
Given the diagnosis of right atrial thrombus in a 24-year-old woman, possible embolic sources were investigated. Venous Doppler ultrasound of the lower limbs revealed patent venous axes. Genetic study showed her to be homozygous for the 675G>A (4G/4G) allelic variant of plasminogen activator inhibitor-1 (PAI-1) and heterozygous for the allelic variant A1298C of 5,10-methylenetetrahydrofolate reductase (MTHFR). No abnormalities were detected in other coagulation factors.
As the thrombus was probably secondary to homozygosity for the 675G>A (4G/4G) allele of PAI-1, the patient has been under anticoagulation therapy ever since.
During 1-year follow-up, following suspension of oral anticoagulation for right inferior lobectomy due to extensive bronchiectasis, she suffered an episode of massive pulmonary embolism with cardiorespiratory arrest.
The subsequent transthoracic echocardiogram revealed no left ventricular dilatation or wall thickening, and good global systolic function; paradoxical septal motion due to right ventricular overload; slight dilatation of the right chambers; and moderate tricuspid regurgitation with estimated pulmonary artery systolic pressure of 54mmHg.
The patient currently experiences a degree of fatigue on moderate exertion but no evidence of embolic phenomena.
In the light of her other prothrombotic risk factors (smoking, morbid obesity and oral contraceptive use), the patient has suspended oral contraceptives, has quit smoking and is taking dietary advice.
DiscussionFree-floating right atrial thrombi are generally rare but are associated with high mortality due to their potential to cause pulmonary thromboembolism.1–3
They are classified according to their origin, as type A when they originate in the deep peripheral veins and as type B when they arise in situ in the right cardiac chambers. Type A are frequently highly mobile and lobulated and are associated with a high incidence of pulmonary thromboembolism, and thus require urgent surgery or thrombolysis. Type B thrombi are often immobile and parietal, and are associated with better prognosis.5 Despite these different prognoses, their management is still the subject of debate.1,2
In a series of 38 consecutive patients diagnosed with free-floating thrombi in the right cardiac chambers, Chartier et al. found that irrespective of the therapeutic strategy adopted (surgery, pharmacological thrombolysis, anticoagulation with heparin or percutaneous treatment), in-hospital mortality was high (44.7%). Nevertheless, they concluded that urgent surgery is normally required and that fibrinolysis can act as a therapeutic bridge for these patients.1
In an analysis covering 34 years, Rose et al. reported that right heart thromboemboli are associated with high mortality (27.1%), and that thrombolysis led to better survival than anticoagulation or surgery, although the authors concluded that prospective randomized studies were needed to determine the best therapeutic approach.2
The case presented is of a patient who, following episodes suggestive of pulmonary thromboembolism, was diagnosed by echocardiography with a right atrial mass. Given the recurrent episodes of pulmonary thromboembolism and a worsening clinical setting, urgent surgery was performed to remove a highly mobile, multilobulated, pedunculated mass adhering to the Eustachian valve, which was confirmed as a thrombus only after anatomopathological study.
An unusual aspect of this case was the fact that this type B thrombus was free-floating.
Given the prognostic implications of a right atrial thrombus, the patient was referred for genetic study, which showed her to be homozygous for the 675G>A (4G/4G) allelic variant of PAI-1 and heterozygous for the allelic variant A1298C of MTHFR.
The relationship between thromboembolic phenomena and the 4G/4G and 4G/5G polymorphism of PAI-1 is becoming clearer. Studies have demonstrated an increased incidence of deep vein thrombosis in carriers of the 4G/5G variant,6 and of thrombotic phenomena, particularly in vessels of internal organs, in carriers of both variants.7
Genetic study also showed the patient to be heterozygous for the allelic variant A1298C of MTHFR, an enzyme involved in homocysteine metabolism, high levels of which are frequently associated with increased risk for ischemic heart disease and deep vein thrombosis. Two polymorphisms on chromosome 1, C677T and A1298C, can increase homocysteine production.8 Although there is a known relation between hyperhomocysteinemia and cardiovascular risk, the pathophysiological mechanisms involved are not fully understood. Unlike A1298C, only homozygosity for the C677T polymorphism is associated with hyperhomocysteinemia. None of the allelic variants presents a higher risk of ischemic heart disease or deep vein thrombosis than healthy individuals without these variants.9,10
The present case illustrates that even in the presence of modifiable risk factors (smoking, morbid obesity and oral contraceptive use) that could explain the clinical presentation, a free-floating right atrial thrombus, or any deep vein embolism, in a young patient should arouse suspicion of a genetic predisposition that, combined with environmental factors, can lead to the occurrence of embolic phenomena with serious therapeutic and prognostic implications.
According to searches in Medline and Google, this is the first case of a right atrial thrombus secondary to homozygosity for the 675G>A (4G/4G) allele of PAI-1.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cordeiro Piçarra, B. Trombo na aurícula direita: Apresentação rara da deficiência do inibidor do ativador do plaminogénio. Rev Port Cardiol; 2012. doi:10.1016/j.repc.2011.12.001.