With expanding indications for cardiac implantable electronic devices (CIEDs) capable of treating bradycardias, complex cardiac tachyarrhythmias and heart failure, the number of patients requiring regular long-term specialized care is growing rapidly. Currently, routine face-to-face follow-up consultations for patients with CIEDs are a significant burden on hospital services. Remote telemonitoring appears to offer a safe and effective alternative to conventional follow-up in this area. The Medtronic CareLink Network enables remote monitoring of CIED patients, and thus has the potential to improve the efficiency of medical care in this population. The objective of the PORTLink (PORTuguese Research on Telemonitoring with CareLink) multicenter randomized trial is to assess the safety, efficacy and costs of remote CIED monitoring compared to traditional face-to-face follow-up. It will evaluate aspects such as physicians’ and patients’ acceptance of and satisfaction with reviewing device data via the website, the complexity for troubleshooting calls to the support center, the use of emergency resources by symptomatic patients, the incidence of unscheduled consultations after remote interrogations, levels of anxiety, depression and quality of life, and the main resources used by the CareLink system. Approximately 200 patients will be randomized in up to five centers, with clinical follow-up of 12 months. Enrollment began in 2012 and is expected to be completed in early 2014.
Com a expansão das indicações para terapêutica com dispositivos cardíacos implantáveis (DCI), capazes de tratar bradiarritmias, taquidisritmias ventriculares e insuficiência cardíaca, o número de doentes que necessitam de seguimento especializado regular a longo prazo tem vindo a aumentar rapidamente. Atualmente, as consultas de rotina com portadores de DCI envolvendo equipas multidisciplinares representam uma sobrecarga significativa na atividade hospitalar. Neste contexto, a monitorização à distância tem sido sugerida como uma opção segura e eficaz, com grande potencial como alternativa ao seguimento convencional. O sistema Medtronic CareLink tem sido largamente implementado na monitorização à distância, podendo associar-se a melhoria na eficiência dos programas de seguimento desta população. O objetivo do PORTuguese Research on Telemonitoring with CareLink (PORTLink), um estudo multicêntrico randomizado, é avaliar a segurança, a eficácia e os custos da monitorização à distância de DCI, quando comparados com o seguimento hospitalar convencional. O estudo pretende avaliar aspetos como a aceitação e satisfação da equipa médica e doente com os dados do funcionamento do dispositivo obtidos via website, a complexidade referida pelos vários centros na deteção e resolução de problemas, a utilização dos recursos de urgência por doentes sintomáticos, a incidência de consultas não programadas, os níveis de ansiedade, depressão e qualidade de vida, e o consumo de recursos associados ao funcionamento do sistema CareLink. Serão aleatorizados cerca de 200 doentes em até cinco centros, com seguimento clínico de 12 meses. A inclusão de doentes iniciou-se em 2012 e tem conclusão prevista para o início de 2014.
The last decade has seen a significant increase in the use of cardiac implantable electronic devices (CIEDs) as a consequence of the demonstrated benefits of implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy defibrillator (CRT-D) devices in reducing mortality in selected patients.1,2 Between 1990 and 2002 the number of ICDs implanted in the US grew more than 10-fold,3 while a similar increase was seen between 2000 and 2010 in Portugal, reaching 100 devices per million population in 2010, approaching the European average of 150 per million in 2007.4,5 However, it has been suggested that not all patients eligible for an ICD receive this therapy, which means that if all potential candidates were to be implanted, the hospital resources available for clinical follow-up of these patients would be overwhelmed and unable to cope.6,7 Growth in this area has already had an impact on management of the resources required to deal with the increasing numbers of patients needing specialized follow-up consultations.1,2 The task of monitoring the operating parameters of the different devices, including identification and resolution of problems, detection and treatment of arrhythmias, and ensuring reliable biventricular stimulation, as well as providing other specialist clinical care, requires a trained and skillful hospital team. Furthermore, in most cases follow-up consultations for CIEDs are scheduled at 3- to 6-month intervals.8–10 If the population with CIEDs continues to expand, this will lead to an exponential increase in the number of follow-up visits, placing enormous strain on hospitals’ resources, including specialist human resources, which in this area of cardiology are relatively scarce.
Against this background, the use of remote monitoring systems has become a topic of much debate. Telemedicine systems for remote monitoring of CIEDs are already available and are changing the follow-up of these patients; their benefits and safety are well documented.10–12 However, certain questions, both clinical and technical, arising from the increasing use of these systems in clinical practice, need to be addressed. The PORTLink (PORTuguese Research on Telemonitoring with CareLink) trial sets out to assess whether the CareLink remote monitoring system (Medtronic Inc., Minneapolis, MN, USA) improves the efficiency of follow-up in patients with CIEDs in terms of safety, efficacy, patients’ and physicians’ satisfaction, and resource use, compared to traditional face-to-face follow-up.
Methods and study designPORTLink is a controlled, non-blinded, multicenter prospective randomized clinical trial with parallel groups in Portuguese hospitals. The study population will be enrolled between 2012 and 2014, 200 patients being randomized and followed for 12 months.
Patient selection and randomizationTable 1 shows the trial's inclusion and exclusion criteria. Eligibility will be confirmed during the enrollment period in consultations with patients with CIEDs.
Criteria for participation in the PORTLink trial.
| Inclusion criteria | - Patients with CIEDs followed in face-to-face consultations- Patients with Medtronic ICD or CRT-D- Able to use the CareLink service according to assessment by a specialist and willing to use it- Provision of written informed consent |
| Exclusion criteria | - Any clinical condition that limits participation in the trial- Those with an ICD compatible only with the non-wireless CareLink 2490G monitor and without telephone access- Age under 18- Participating in another clinical trial |
| Study withdrawal | - Patients who change to a different hospital for CIED follow-up- Occurrence of severe incapacity with no possibility of support from family or healthcare provider- Death |
Medtronic devices compatible with the CareLink 2490G monitor: Marquis, Maximo, Entrust and Intrinsic (ICDs), and InSync Marquis, InSyncII Marquis, Marquis, InSync Maximo and Insync Sentry (CRT-Ds). Devices compatible with the CareLink 2490C monitor: iVirtuoso, Maximo II, Virtuoso II, Secura, Concerto, Concerto II and Consulta. Abbreviations as in text.
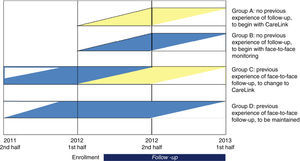
After enrollment, eligible patients will be allocated randomly in a 1:1:1:1 ratio to one of four groups: group A, with a newly implanted CIED and no previous experience of follow-up, who will begin remote monitoring with the CareLink system; group B, with a newly implanted CIED and no previous experience of follow-up, who will begin traditional monitoring in face-to-face consultations; group C, with experience of monitoring in face-to-face consultations, who will begin remote monitoring with the CareLink system; and group D, with experience of monitoring in who will maintain this monitoring protocol.
Follow-upAt the first outpatient visit after randomization and provision of informed consent, patients will be advised of what type of follow-up they will receive, and patients in groups A and C will be informed about how the CareLink system functions and the number of scheduled transmissions during the 12-month follow-up. Those in groups B and D, with traditional face-to-face follow-up, will have three or four scheduled visits during the 12-month period.
In accordance with the Heart Rhythm Society/European Heart Rhythm Association (HRS/EHRA) guidelines on the monitoring of CIEDs,10 the device will be monitored within 72 hours of implantation (face-to-face), 2–12 weeks after implantation (face-to-face), and then every 3–6 months (face-to-face or remote). Patients with remote monitoring will undergo annual face-to-face assessment.
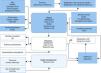
The Medtronic CareLink systemThe Medtronic CareLink system for remote monitoring of Medtronic CIEDs combines computer and biomedical technology to create an interface for transferring data from the device to the clinic. It has been used in various Portuguese hospitals since 2009 and hundreds of patients are now being followed by this method (Figure 1).
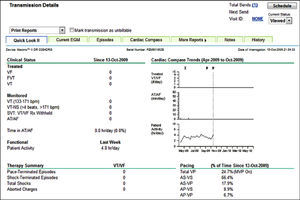
The system consists of the CareLink Monitor for use by the patient, which transfers data from Medtronic CIEDs (Figure 2), and the CareLink software used by the team responsible for follow-up. The device can be interrogated manually using a wand linked to the monitor in the patient's home, or automatically using wireless systems. Data can be scheduled to be transmitted regularly and can be sent when clinical circumstances dictate, by agreement between the patient and the team. The data are transmitted to a central (internet-based) data repository, access to which is limited, each center only having access to data on its own patients through a password-protected web page. Members of the hospital team can access the CareLink program in order to consult and analyze the data transmitted by the monitor, including changes in device parameters, arrhythmic episodes recorded on intracavitary electrograms, therapies delivered by the device, the percentage of different pacing modes and tachyarrhythmias treated (Figure 3).
Specific objectives and criteriaThe PORTLink study sets out to assess the level of satisfaction, clinical benefit, safety and resource use of remote monitoring with the Medtronic CareLink Network in patients with CIEDs compared to traditional face-to-face follow-up. The following criteria will be used to compare the different follow-up protocols:
- •
the proportion of patients satisfied with their monitoring protocol
- •
the proportion of adverse events identified
- •
the proportion of unscheduled face-to-face consultations and the reasons for them
- •
the proportion of resources consumed from the standpoint of the patient and of the National Health Service (NHS).
Other criteria to be assessed in the remote monitoring groups are the proportion of successful transmissions without need for additional telephone contact, and ease of use of and level of satisfaction with the CareLink service from the standpoint of physicians and health workers.
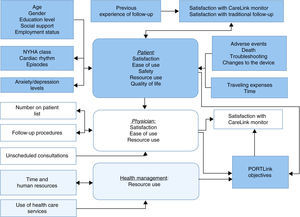
Study variables and data collectionFigure 4 presents the underlying conceptual model of the PORTLink trial and the variables to be analyzed. Data will be collected through forms and questionnaires in face-to-face consultations and following each remote transmission.
At the time of enrollment and baseline assessment, the physician will explain the study's characteristics to the patient and request informed consent, and will fill out a form recording data on symptoms, underlying disease, comorbidities, previous treatment, history of arrhythmias and type of CIED.
The following will be applied to the patient:
- •
a questionnaire on education level, social support and resources used in travel to and from hospital;
- •
the Hospital Anxiety and Depression Scale (HADS), validated in Portuguese by Pais-Ribeiro et al.13;
- •
the 12-Item Short-Form Health Survey (SF-12), which measures perception of health-related quality of life, with a physical component summary and a mental component summary.14
In this initial phase of the study, patients in groups A and C will be receive instructions about the operation of the CareLink system and transmissions will be scheduled for the 12 months of follow-up, and face-to-face consultations will be scheduled for those in groups B and D, with traditional follow-up.
For patients allocated to remote monitoring, patient and physician will complete questionnaires after each scheduled transmission. The patient's questionnaire will record information on his or her level of satisfaction with the system, while the physician's will cover technical matters including the occurrence and characterization of arrhythmias and other relevant clinical events, the quality of the data transmitted, time taken to analyze the data, the need for intervention after assessment of the data, and level of satisfaction with the system. For unscheduled transmissions, the reason for the transmission will be recorded, as well as any resulting changes in therapy or device programming, or need for hospital visits.
Patient and physician questionnaires will also be used during face-to-face follow-up consultations to enable comparison between the traditional and remote monitoring protocols. These questionnaires will collect data as follows:
- •
for patients, information on the type of consultation (scheduled or unscheduled), time spent traveling to and from the hospital, type of transport used and distance traveled, need to be accompanied, waiting and consultation time, extent of disruption to the patient's and any companion's daily routine (particularly time off work), and degree of satisfaction with the consultation.
- •
for physicians, information on analysis of CIED data, occurrence and characterization of arrhythmic episodes, members of the team involved in the consultation, type of intervention performed after assessment of the device parameters, and time spent on consultations (for both face-to-face and remote monitoring).
At the final assessment, after 12 months of follow-up, both physician and patient will fill out forms assessing their level of satisfaction and recording clinical status, arrhythmic episodes and CIED parameters. A face-to-face questionnaire and the HADS and SF-12 scales will again be applied to all patients.
During the study, when applicable, forms will be filled out to record any adverse clinical events (whether causally related to the CIED or not), study withdrawal, problem resolution, and any deviation from the trial design.
The questionnaires will provide data on resource use for follow-up by both the NHS and the patient, enabling comparison of the mean number of face-to-face consultations, both scheduled and unscheduled; the total number of consultations (for both groups); and the mean time spent by physicians in both types of follow-up. For patients, the time spent in face-to-face consultations, distance traveled, and time off work (for the patient and companion, if any) will be quantified. This information will be used to calculate costs according to official NHS and Government methods to enable an economic comparison between traditional face-to-face follow-up and remote monitoring.30,31
Statistical powerThe statistical methods and determination of the study's statistical power, and hence the sample size, are defined on the basis of the trial's objectives and the characteristics of the four groups. The level of significance is set at 95% for each analysis, with a beta error of 0.20. Descriptive data and comparisons between groups will be presented based on clinical variables, device parameters and data collected in face-to-face consultations and remotely, and information from the various questionnaires applied during the study.
Assuming that individuals undergoing CareLink remote monitoring (groups A and C, n=100) and those with traditional follow-up (groups B and D, n=100) are two independent groups, it will be possible to identify differences between dichotomous variables with a prevalence of 50% in the traditional follow-up groups that are associated with remote monitoring with a relative risk of ≥1.39 or ≤0.61. It will also be possible to compare individuals with and without previous experience of face-to-face follow-up in terms of levels of satisfaction with their monitoring protocol. In groups A and C (remote monitoring) physicians’ levels of satisfaction will also be assessed, and a prospective comparison will be made on ease of use and satisfaction, information from scheduled and unscheduled transmissions and consultations, transmission times, and use of other resources. Assuming each group consists of a minimum of 50 individuals and that for comparisons between remote and traditional follow-up the groups are independent, it will be possible to identify differences between dichotomous variables with a prevalence of 50% in the traditional follow-up groups that are associated with remote monitoring with a relative risk of ≥1.53 or ≤0.47.
Comparison between baseline and final data for each group, assuming a minimum of 50 individuals in each group, is paired, and it will be possible to identify differences between dichotomous variables with a prevalence of 50% on baseline assessment and a correlation coefficient of 0.5 that are associated with remote monitoring with a relative risk of ≥1.38 or ≤0.62.
Ethical aspectsThe study protocol is in accordance with the ethical principles of the Declaration of Helsinki and has been approved by the Portuguese National Data Protection Commission, INFARMED and the ethics committees of the participating centers. All participants will provide written informed consent for inclusion in the study. The trial is sponsored by Medtronic Portugal and will be monitored by supervisors from the Institute of Preventive Medicine of Lisbon University Medical School. Confidentiality of personal data will be protected by making it unidentifiable, participants being assigned a randomly generated unique number. The data will be stored in a secure password-protected online database, accessible only to study investigators and supervisors from the Institute of Preventive Medicine. The monitoring committee, made up of the principal clinical investigator from each center and representatives from the Institute of Preventive Medicine, will act as a consulting body and will monitor how the study is performed; it will also have access to the data analysis.
DiscussionAs more individuals receive CIEDs, the population requiring follow-up for these devices is set to increase significantly, particularly in high-volume centers.10,14–17 This follow-up requires regular, long-term, and specialized monitoring, involving periodic hospital visits, in order to ensure that the device is working properly and to intervene in accordance with the patient's clinical course, with particular regard to the occurrence of arrhythmias and/or heart failure.10 However, a retrospective analysis has shown that most face-to-face consultations are in fact routine, with no clinically relevant findings in 78.2% of cases and no changes to treatment or device programming in 90%.18
The implementation of a monitoring system that can maintain safety levels while reducing the costs involved in terms of human and logistical resources is an increasingly attractive option in clinical practice, with advantages in terms of satisfaction, costs, resource optimization and safety that have been demonstrated in several multicenter studies.18–21
The ability of remote monitoring systems to provide regular assessments of the technical status of the different components of the device, to detect and characterize arrhythmias and therapies applied, and to identify variables associated with the risk of hospitalization for decompensated heart failure, has the potential to improve the way that patients with CIEDs are monitored, as shown in studies demonstrating greater efficiency with remote monitoring systems11,22,23 and reductions in overall costs per patient and in the number of hours worked by medical staff. Remote monitoring23–26 also appears to be a safe option, particularly regarding the early detection of device malfunction or changes in the patient's clinical status.12,19,20,25,29 They have also been shown to be feasible and easy to use by both physicians11,27 and patients.11,19,28
However, certain questions remain to be clarified with regard to remote monitoring, particularly concerning safety in cases when the physician wishes to intervene after analyzing the device data but the patient is not contactable, the impact on patients’ quality of life, and assessment of long-term satisfaction. The PORTLink trial sets out to evaluate these aspects by assessing the safety, efficacy, costs, satisfaction, levels of anxiety, depression and quality of life in different groups of patients with CIEDs, comparing remote monitoring systems with the traditional follow-up that is currently the norm in Portugal.
Conflicts of interestMário Oliveira is a member of the European Advisory Board of Medtronic. The other authors have no conflicts of interest to declare.24
Please cite this article as: Oliveira M, Fernandes M, Primo J, et al. Monitorização à distância versus seguimento convencional presencial em portadores de dispositivos cardíacos implantados: racional e desenho do estudo PORTLink (PORTuguese Research on Telemonitoring with CareLink). Rev Port Cardiol. 2013;32:957–964.