The authors present two cases of purulent pericarditis secondary to pneumococcus pneumonia, a rare entity in the antibiotic era, one of them in an apparently healthy person. A systematized diagnostic approach to moderate pericardial effusion is presented, together with a review of purulent pericarditis. The presence of pericardial effusion with persistent fever with or without known etiology, particularly in the immunocompromised but also in the apparently healthy patient, should always raise the possibility of purulent pericarditis.
Os autores apresentam dois casos de pericardite purulenta secundária a pneumonia por pneumococos, um deles num doente sem antecedentes patológicos conhecidos. É feita uma sistematização da abordagem diagnóstica ao derrame pericárdico de moderadas dimensões e uma revisão da pericardite purulenta, uma entidade muito rara na era da antibioterapia. A constatação de derrame pericárdico com quadro de febre persistente, com ou sem origem conhecida, fundamentalmente no doente com compromisso imune, mas também no aparentemente saudável, deve levantar-se sempre a possibilidade de pericardite purulenta.
Pericardial disease mainly manifests as pericarditis and/or pericardial effusion (PE) and is caused by a variety of etiologies, including infection, inflammation and neoplasms, or iatrogenic, traumatic or metabolic origin, or unknown (idiopathic).
It may occur in isolation, or be one component, of lesser or greater clinical importance, in the presentation of entities with systemic involvement.1
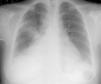
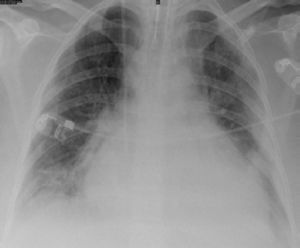
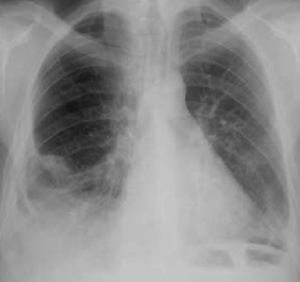
Case reportsCase 1A 39-year-old woman, with no history of disease and not taking regular medication, came to the emergency department for right lower back pain and worsening dyspnea on moderate exertion of one week's duration. She reported no fever, cough or expectoration, and was normotensive, with tachyarrhythmia and mild hypoxemic respiratory failure. Laboratory tests showed signs of inflammation and infection, the ECG revealed atrial fibrillation with rapid ventricular rate (≈115 bpm), and the chest X-ray showed increased cardiothoracic index and segmental infiltrate in the mid third of the right hemithorax (Figure 1).
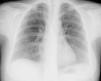
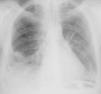
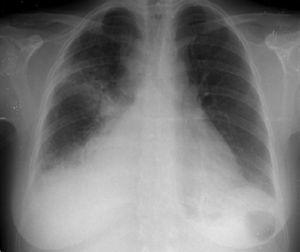
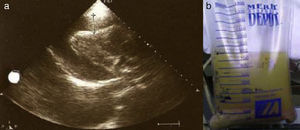
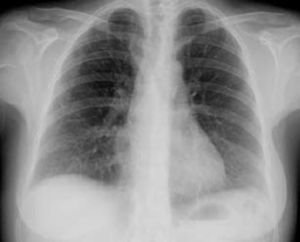
The patient was admitted with a diagnosis of community-acquired pneumonia, and empirical antibiotic therapy was begun with ceftriaxone and azithromycin. Thoracic computed tomography (CT) on the second day of hospitalization showed bilateral lung condensations, bilateral pleural effusion and PE, which on transthoracic echocardiography (TTE) measured 10 mm at the posterior wall in diastole, with no signs of cardiac tamponade. On the same day, there was a sudden deterioration of the clinical setting, evolving to septic shock, and the patient was transferred to the intensive care unit. Inotropic support was required for three days, but chemical cardioversion to sinus rhythm was achieved on the second day. On the third day, multisusceptible Streptococcus pneumoniae was identified in blood samples collected at admission and therapy was begun with penicillin G. Respiratory and hemodynamic stabilization was achieved from the fourth day, although the patient still required mechanical ventilation and remained febrile, with no resolution of the infection on laboratory tests. The cardiac silhouette was still enlarged and serial TTE showed progressive worsening of the PE, which measured 20 mm at the posterior wall in diastole on the 10th day, with signs of cardiac tamponade (Figures 2 and 3A). Pericardiocentesis was therefore performed for diagnostic purposes and drainage, 800 cc of fibrinous purulent fluid being drained (Figure 3B), which was negative on bacteriological study, both direct and in culture. Screening for acid-alcohol resistant bacilli (AARB) was also negative. Subsequent TTE revealed resolution of the effusion, accompanied by clinical, radiological and laboratory improvement (Figure 4). Screening for immune deficiency diseases was negative. The patient presented no constrictive pattern on TTE at 12-month follow-up.
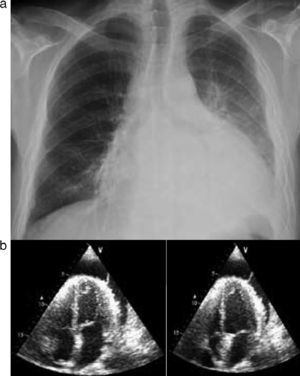
A 33-year-old man had a history of congenital hypogammaglobulinemia (diagnosed in 2001), chronic alcoholism and recurrent pneumonia, the last episode (in 2005) having been complicated by pleural effusion requiring decortication. He had taken no medication since 2005. He came to the emergency department for fever and worsening dyspnea over the previous five days. At admission, he was febrile, prostrate, tachycardic and hypotensive. Laboratory tests showed marked elevation of inflammatory and infectious parameters and arterial blood gas analysis revealed hypoxemic respiratory failure. A chest X-ray showed a markedly enlarged cardiac silhouette, with no parenchymal lung lesions; TTE was therefore performed, which confirmed the presence of a PE, measuring 23 mm at the posterior wall in diastole, with abnormal motion and diastolic collapse of the right atrial free wall (Figure 5A and B).
In view of the setting of septic/obstructive shock, the patient was transferred to the intensive care unit, and diagnostic and therapeutic pericardiocentesis was performed, 700 cc of purulent fluid being drained (Figure 6).
Thoracic CT following pericardiocentesis showed a moderate PE, a small right pleural effusion and an area of parenchymal condensation in the superior and posterior segments of the left lower lobe, with air bronchograms, highly suggestive of inflammation.
The patient remained in cardiocirculatory shock and required inotropic support until the seventh day of hospitalization. Initially, the fluid drained was purulent, and then became serofibrinous. On the ninth day, the absence of pericardial fluid and evidence of a considerable reduction in the effusion (to 3 mm) at the posterior wall in diastole led to removal of the drain. Empirical antibiotic therapy with ceftriaxone and vancomycin had been begun at admission, which was changed to penicillin G on the third day following confirmation of multisusceptible Streptococcus pneumoniae bacteremia. The presence of pneumococcus with the same susceptibility was confirmed in pericardial fluid, and screening for AARB was negative. Immunoglobulin was administered for four days, which was well tolerated and without complications. Fever returned on the 14th day, and TTE revealed a 15-mm PE at the posterior wall in diastole; repeat thoracic CT showed loci of pleural empyema. The patient was transferred to the thoracic surgery department of Coimbra University Hospitals, where he underwent surgical drainage and decortication of both pleurae, pericardiotomy and creation of a pleuropericardial window, resulting in considerable improvement in clinical and laboratory parameters (Figure 7).
The patient presented no constrictive pattern on TTE at 12-month follow-up.
DiscussionPericardial effusionPE may develop as a result of pericarditis or arise as an epiphenomenon of greater or less clinical significance in a range of systemic disorders. The etiology of moderate PE is idiopathic in 29% of cases, but can be iatrogenic (16%), or due to neoplasms (13%), myocardial infarction (8%), uremia (6%), connective tissue or thyroid disease (5%) or infection (2%).2
The etiological distribution of PE of acute presentation with cardiorespiratory symptoms is different: neoplasm (33%), idiopathic (14%), acute pericarditis (12%), trauma (12%), uremia (6%), post-pericardiotomy (5%) and infection (5%, 4% bacterial).3
It is essential to exclude PE in the following clinical contexts:
- 1.
acute pericarditis;
- 2.
enlarged cardiac silhouette without pulmonary congestion;
- 3.
severe hemodynamic deterioration following an acute coronary event, heart surgery or invasive cardiac procedure; and
- 4.
persistent unexplained fever, whether or not the cause is determined.
A systematic approach to PE involves three stages4:
- 1.
confirmation by TTE;
- 2.
assessment of hemodynamic repercussions (covering a wide spectrum of severity from benign to life-threatening, depending on its volume, the rate at which the effusion accumulates and pericardial elasticity); and
- 3.
determination of etiology.
With regard to etiological determination, certain clinical contexts justify a non-invasive approach, including recent myocardial infarction, hypothyroidism and renal failure. In other cases, diagnosis is based on clinical evaluation as well as biochemical and bacteriological study of pericardial fluid or of the pericardium itself.
Pericardiocentesis has limited diagnostic value (6% of cases) in acute pericarditis. In patients with large chronic effusions, analysis of pericardial fluid leads to a definitive diagnosis in 36% and a probable diagnosis in 40%.5
Pericardiocentesis guided by echography or fluoroscopy is formally indicated6 in cases of:
- 1.
cardiac tamponade;
- 2.
effusion of >20 mm that does not respond after a week of conservative treatment with non-steroidal anti-inflammatory drugs; and
- 3.
clinical suspicion of purulent, tuberculous or neoplastic pericarditis.
Purulent pericarditis is defined as an infection in the pericardial space that produces macroscopically or microscopically purulent fluid. It may be primary (extremely rare) or secondary to another infectious process. There are five pathogenic mechanisms that can lead to invasion of the pericardial space in secondary purulent pericarditis7:
- 1.
contiguous spread from an intrathoracic site;
- 2.
hematogenous spread;
- 3.
extension from a myocardial site;
- 4.
perforating injury or surgery;
- 5.
extension from a subdiaphragmatic site.
Pneumococcus is more commonly associated with contiguous spread from an intrathoracic site, while Staphylococcus aureus is more often involved in hematogenous spread.
Purulent pericarditis is nowadays very rare, occurring most frequently in individuals with previous pericardial disease treated by chemotherapy, and in those who have undergone cardiac surgery or are receiving dialysis; immunosuppression, alcoholism and chest trauma are predisposing factors.5 Before the advent of antibiotic therapy, it was a common complication of pneumonia, endocarditis, meningitis and other infections of varying severity, including of bone, skin and the ear.7
A retrospective analysis of a Spanish hospital population of 593 601 patients between 1972 and 1991 found 33 cases of purulent pericarditis, of which only 19 (57%) were diagnosed in life, basically because the diagnosis was not considered.8Table 1 presents a review of the literature, covering etiology, bacteriology, associated comorbidities, treatment and outcome.
Purulent pericarditis: brief review of the literature.
| Study | Number of patients | Comorbidities | Origin of infection | Bacteriology | Treatment | Outcome |
| Sagrista-Sauleda et al.8 | 33 | Alcoholism, rheumatoid arthritis, ulcerous colitis (n=5) | Pneumonia (n=10)Peritonsilar abscess, cervical abscess, mediastinitis (n=5)Sepsis (skin, oral cavity, parenteral nutrition, colon cancer) (n=4)Periodontal infection, oral abscess, mediastinitis (n=3)Biliary tract infection (n=3)Urinary tract infection (n=2)Catheter-related sepsis, including temporary pacemaker (n=2)Post-traumatic subphrenic abscess (n=1)Meningitis (n=1)Mastoiditis (n=1)Liver transplant (n=1) | Peptococcus sp., Gram-negative, anaerobic (n=1)Streptococcus milleri (n=1)Streptococcus pneumoniae (n=2)Streptococcus mitis, Bacteroides sp., Gram-negative, anaerobic (n=1)Staphylococcus aureus (n=2)Clostridium septicum (n=1)Pseudomonas aeruginosa (n=2)Escherichia coli (n=1)Staphylococcus aureus, S. mitis, P. aeruginosa (n=1)Klebsiella sp., E. faecalis, Proteus sp. (n=1)E. coli, Klebsiella sp. (n=1)E. coli, anaerobic (n=1) | Pericardiocentesis followed by pericardiectomy (n=12)Pericardiectomy as initial approach (n=4)Pericardiocentesis (n=2)Post-mortem diagnosis (n=14) | Death (n=3, due to renal failure n=1, complications due to pericardial disease n=2) |
| Kauffman et al.9 | 118 | Chronic alcoholism (n=2), hypogammaglobulinemia (n=1) | Pneumonia (n=81)Pneumonia and empyema (n=58)Otitis media (n=6)Arthritis (n=2)Subcutaneous abscess (n=2)Meningitis (n=1)Endocarditis (n=1)None identified (n=2)NB Some patients had more than one site of infection. | S. pneumoniae (all patients) | None (n=52)Pericardiectomy (n=49)Pericardiectomy + antibiotic therapy (n=10)Pericardiocentesis (n=1)Pericardiocentesis + antibiotic therapy (n=1) | Death (n=64, including all who received no treatment, 19 who underwent pericardiectomy only, and one who underwent pericardiocentesis only) |
| Rubin et al.7 | 26 | Liver failure (n=2)Uremia (n=1)Diabetes (n=3)Leukopenia (n=1)Third degree burns (n=2)Acute lymphoblastic leukemia (n=1)Iatrogenic immunosuppression (n=1) | Esophageal perforation following thoracic surgery (n=2)Wound infection following thoracic surgery (n=6)Pneumonia (n=6)Mediastinal abscess (n=1)Infective endocarditis (n=3)Intramyocardial abscess (n=2)Bacteremia (n=6) | S. aureus (n=8)S. pneumoniae (n=2)Haemophilus influenzae (n=2)Neisseria meningitidis (n=1)P. aeruginosa (n=1)Bacteroides spp. (n=1)Salmonella typhimurium (n=1)Nocardia asteroides (n=1)S. mitis (n=1)Mixed bacterial infections (n=2)Candida spp. (n=5) | Antibiotic therapy + pericardiocentesis (n=11)Antibiotic therapy without pericardiocentesis (n=15) | Death (n=15, patients who received antibiotic therapy only) |
| Klacsmann et al.10 | 200 | Chronic renal disease (n=16)Cancer (13)Acute myocardial infarction (n=2)Diabetes, myeloproliferative disease (n=10)Sickle-cell anemia (n=3) | Pneumonia (n=104)Bacteremia (n=44)Endocarditis/myocardial abscess (n=30)Perforating chest injury (n=18)Suppurative subdiaphragmatic lesion (n=4) | Pneumococcus sp. (n=69)Staphylococcus sp. (n=38)Streptococcus sp. (n=21)Proteus sp., E. coli, Pseudomonas sp., Klebsiella sp. (n=25)Salmonella/Shigella (n=4)N. meningitidis (n=5) | Post-mortem diagnosis (n=200) | Death (all patients) |
A patient with sepsis and a known underlying disease that explains the presence of PE makes prompt diagnosis of purulent pericarditis more difficult.2 The diagnosis can only be confirmed by pericardiocentesis (which in such cases in therapeutic) by means of macroscopic examination of the fluid, which has the biochemical characteristics of an exudate and should be subjected to microscopic study, direct and in culture, to screen for bacteria, fungi and AARB.
Treatment must include drainage of the pericardial space, combined with systemic antibiotic therapy, initially empirical (vancomycin and ceftriaxone or imipenem, meropenem or piperacillin-tazobactam, together with fluconazole in immunocompromised patients), and then adjusted according to the results of microbiological study; local antibiotic therapy confers no benefit. Antibiotic therapy should be continued for at least 28 days, or until fever has subsided and there are no laboratory signs of infection.
The strategy adopted to achieve complete drainage of the pericardial space will depend on the human and technical resources of the institution treating the patient6,11:
- 1.
Pericardiocentesis. This is the simplest and fastest method, but is often ineffective in draining thick, loculated fibrinous fluid. It also the technique that most frequently leads to development of constrictive pericarditis. Intrapericardial infusion of fibrinolytics can increase its therapeutic efficacy but is associated with complications, and is thus not generally recommended.12
- 2.
Pericardiotomy (creating a pericardial window, subxiphoid if an isolated procedure). This is the method recommended in the European Society of Cardiology guidelines, since it is associated with a higher success rate and a lower incidence of constrictive pericarditis.
- 3.
Pericardiectomy. This is associated with mortality of 8% but it is the approach than resolves all situations, even the most complicated (adhesions, loculated effusions or persistent infection).6
Without drainage of the pericardial space, purulent pericarditis leads inexorably to death. Mortality in patients who are promptly diagnosed and appropriately treated is 40%, generally due to cardiac tamponade, septic shock or constriction. Mortality increases the longer diagnosis and treatment are delayed, and is higher in those with S. aureus infection and in malnourished patients.11
ConclusionsThe interest of these two cases resides mainly in the question of the approach to adopt in a septic patient with PE. Effusions with persistent fever, of known or unknown origin, should always raise the possibility of purulent pericarditis. This is true for both immunocompromised patients and previously healthy individuals who, even if they have no predisposing condition, have the same risk if they develop pneumonia – the main cause of purulent pericarditis identified in various series.2,7–10 In addition, although a diagnosis of purulent pericarditis is more likely the closer the primary infection site is to the pericardium, septic emboli from a suppurative site can lead to pericardial, myocardial or mediastinal spread through bronchial arteries without involving the rest of the systemic circulation,7 which was the most likely mechanism in the first case presented.
Prompt diagnosis of purulent pericarditis and initiation of appropriate treatment are the mainstays of successful management of this rare but potentially lethal entity.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Pericardite purulenta: um diagnóstico raro. Rev Port Cardiol. 2013;32:721–727.