Coronary occlusion is one of the potential complications during transcatheter aortic valve implantation. Second-generation fully retrievable and repositionable devices can theoretically prevent this complication.
A 81-year-old woman with symptomatic aortic stenosis (valve area 0.52 cm2; peak and mean gradients 118 and 70 mmHg, respectively; normal ejection fraction) was referred for transcatheter aortic valve implantation. Although her surgical risk was acceptable (logistic euroSCORE 8%), open surgery was rejected due to porcelain aorta. Preprocedural assessment included coronary angiography (normal coronary arteries) and cardiac computed tomography (aortic annulus 21 mm).
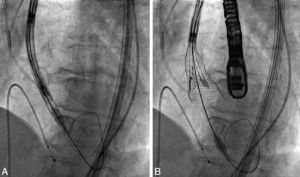
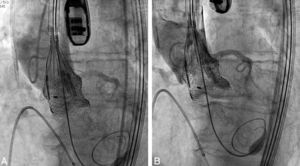
A 23 mm Lotus device (Boston Scientific) was implanted. After an 18 F sheath was advanced through the right femoral artery, percutaneous aortic valvuloplasty was performed using an 18 mm balloon and a Safari guidewire. The delivery system and the unexpanded valve were advanced retrogradely to the left ventricular cavity (Figure 1A) and manually controlled expansion of the valve was performed (Figure 1B).
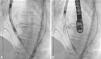
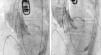
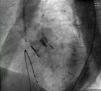
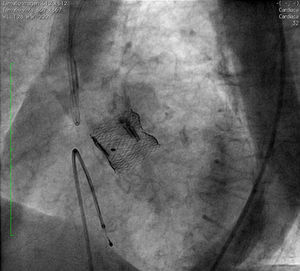
After complete expansion of the valve, the aortic angiogram showed apparent occlusion of the left main due to the slightly high position of the valve (Figure 2A). Due to the possibility of resheathing the Lotus device even after it had been completely expanded, the valve was retrieved and repositioned in a lower position, without compromising the left main (Figure 2B), thus allowing final release of the valve (Figure 3).
This case illustrates the potential advantages of second-generation devices for transcatheter aortic valve implantation. With the Lotus device, retrieval and repositioning are possible even when the valve has been completely expanded and positioned.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.