Atrial fibrillation is a common arrhythmia in clinical practice. It is associated with high morbidity and mortality due to its thromboembolic potential, which makes thromboembolic prevention particularly important. Warfarin has been the first-line therapy for this purpose, but it has various limitations and is often contraindicated or underutilized. The fact that thrombi are frequently located in the left atrial appendage in atrial fibrillation led to the development of percutaneous closure for thromboembolic prevention. This article examines the current evidence on percutaneous closure of the left atrial appendage by reviewing the results of the numerous clinical trials on the technique.
A fibrilhação auricular é uma arritmia frequente na prática clínica. Associa-se à morbilidade e à mortalidade elevadas, em virtude do seu potencial tromboembólico, o que confere especial relevância à profilaxia do tromboembolismo. Para este efeito, a varfarina tem sido a terapêutica de primeira linha, no entanto, associa-se a inúmeras limitações, que a tornam contraindicada ou sub-utilizada. A localização frequente no apêndice auricular esquerdo dos trombos formados na fibrilhação auricular conduziu a que o seu encerramento percutâneo fosse desenvolvido para profilaxia do tromboembolismo. Este artigo pretende fazer um enquadramento da evidência atual para o encerramento percutâneo do apêndice auricular esquerdo, através de uma revisão e atualização dos resultados dos inúmeros estudos realizados até ao momento.
Atrial fibrillation (AF) is a common arrhythmia with high morbidity and mortality due to its thromboembolic potential. Warfarin has been the first-line therapy for thromboembolic prevention, but it has various contraindications and limitations. Thrombi in AF form mainly in the left atrial appendage (LAA), and so closure of the LAA is considered an alternative to warfarin therapy.
Prevalence of atrial fibrillationAF is the most common cardiac arrhythmia in clinical practice. Its prevalence in Portugal is 2.5% in those aged 40 or over according to the FAMA study.1 The figure in the general population is 1–2%, rising with age; prevalence has increased significantly over time and is predicted to double in the next 50 years.2
The importance of thromboembolism in atrial fibrillationThe risk of stroke is five times higher in those with AF than in those in sinus rhythm.2,3 The large size of the thrombi that cause these strokes means that their consequences tend to be more severe than from other sources of cerebral thrombi.2,4–6
Chronic anticoagulation with warfarinOral anticoagulation with warfarin remains the first-line therapy to prevent thromboembolic events in AF. It is indicated in all patients with CHADS2 or CHA2DS2-VASC score ≥2, and should also be considered with a score of 1.2 Its efficacy has been demonstrated in numerous randomized clinical trials, which have shown that with a target international normalized ratio (INR) of 2–3, relative risk for stroke is reduced by 60–73%.2,4–9 Its superiority over aspirin (reduction of 20%2,7), combined aspirin and clopidogrel, and a single antiplatelet drug plus low-dose warfarin,2,10,11 is well established.
However, warfarin is contraindicated in 14–44% of patients at risk of stroke.12 Even among eligible patients, only 54% are anticoagulated, for a variety of reasons, the most important being bleeding risk; others include a narrow therapeutic window and the sensitivity of its pharmacokinetics to a range of foods and other drugs, which necessitate frequent laboratory testing and the patient's cooperation. The risk of trauma, access to INR monitoring, clinicians’ wariness, and patient preferences can also make warfarin therapy impractical.4 Anticoagulation is thus often inadequate, and INR values are within the therapeutic window in only 50–68% of tests.9
New oral anticoagulantsNew anticoagulants have been developed as alternatives to warfarin for thromboembolic prevention in AF. There are two main classes: direct thrombin inhibitors such as dabigatran, and factor Xa inhibitors such as rivaroxaban, apixaban, edoxaban and betrixaban. They all have advantages over warfarin including a wider therapeutic window, fewer interactions with foods, and no need for laboratory monitoring.2 Only dabigatran (the RE-LY study13), rivaroxaban (ROCKET-AF14) and apixaban (ARISTOTLE15) have demonstrated non-inferiority to warfarin in thromboembolic prevention in AF, and only the first two have been approved by the US Food and Drug Administration (FDA) for this purpose.16 The latest guidelines for the management of atrial fibrillation of the European Society of Cardiology consider dabigatran an alternative in patients at high embolic risk if warfarin is contraindicated or impractical.2 The latest Canadian Cardiovascular Society guidelines also recommend the use of dabigatran rather than warfarin in AF patients with indication for oral anticoagulation.17
However, these drugs are expensive for chronic therapy, carry a significant risk of bleeding, and do not have an established antidote, all which are obstacles to their use in many patients. Studies on these new anticoagulants have also shown significant rates of discontinuation of therapy, mainly due to intolerance or adverse effects, reaching 25.3% in patients taking apixaban (vs. 27.5% for those taking warfarin) in the ARISTOTLE trial,15 but higher than seen for warfarin in the RE-LY13 (21% for dabigatran vs. 17%) and ROCKET-AF14 (23.7% for rivaroxaban vs. 22.2%) trials.
The importance of the left atrial appendage in thromboembolismThe LAA is an embryonic remnant of the left atrium (LA) consisting of a long tubular body with walls as little as 1mm thick, usually multilobulated and trabeculated, that communicates with the LA through an oval orifice.18,19 It is generally thought to have a role in regulating body volume via physiological mechanisms that include the production of 30% of atrial natriuretic peptide, regulation of thirst, and modulation of the volume/pressure ratio and LA compliance, and hence cardiac output.20–22
The LAA is also the most common site of intracardiac thrombi in AF (98%), as demonstrated in autopsy studies, by transesophageal echocardiography (TEE) and by direct intraoperative inspection.11,12,18,19 It is more frequently the site of thrombi in non-valvular (90%) than valvular AF (57%),12,18,19 and in patients with previous ischemic stroke.20
In the light of these facts, exclusion of the LAA from the circulation was seen as an alternative to pharmacological anticoagulation to prevent thromboembolism in AF. Different methods have been developed, some more invasive than others.12,13
Surgical exclusion of the LAAThe first attempt to surgically exclude the LAA, during mitral valve surgery, was described in the 1930s.5 The LAAOS study, the first randomized trial of surgical LAA occlusion, in patients referred for coronary bypass surgery at risk for AF or ischemic stroke, concluded that surgical exclusion of the LAA was safe and did not increase operative time or perioperative bleeding.23
Surgical exclusion began to be performed in various centers, but it was then discovered that exclusion was often incomplete, and success rates varied widely (10.3–60%22) due to differences in surgical techniques, surgical expertise, and criteria for success.
Kanderian et al.22 retrospectively analyzed TEEs performed after surgical LAA closure, which was not at that time a routine procedure, and found a higher failure rate than those seen in small series, in which the criteria for occlusion were less rigorous. The authors concluded that excision was the most effective technique and that incomplete closure increased the likelihood of blood pooling and thrombus formation; they recommended that anticoagulation should be continued until confirmation of complete occlusion by TEE.
Open surgery for LAA closure via an epicardial approach has also been performed using the AtriCure system (AtriClip, Cincinnati, OH) in AF patients undergoing coronary bypass surgery or valve replacement; the three-month success rate was 100%.13 A thoracoscopic epicardial approach was used for LAA occlusion in 15 patients for thromboembolic prevention, and in many other small series during AF ablation.24 However, this approach was associated with additional complications including pneumothorax and need for conversion to open surgery due to bleeding, adherences, or other problems.24
The invasive nature of surgical LAA occlusion means that it has only been performed during cardiac surgery for another reason. It is used as part of the MAZE procedure5,22 and is recommended in the ACC/AHA guidelines on valvular heart disease during mitral valve surgery.25
Minimally invasive LAA exclusionThe invasive nature of surgical LAA exclusion has prompted the development of closure devices using minimally invasive methods, including techniques combining epicardial and endocardial approaches. One example is the LARIAT® system (SentreHEART, Palo Alto, CA), approved by the FDA, which combines epicardial delivery of a snare with a pre-tied suture via a small-caliber catheter and a balloon catheter introduced endocardially using a magnet-tipped guidewire to position the suture around the base of the LAA. The system was assessed in AF patients undergoing mitral valve surgery (n=2) or AF ablation (n=11), and its feasibility was demonstrated.26
Percutaneous LAA closure techniques have also been developed that use an exclusively endocardial approach.
Endocardial percutaneous LAA closurePercutaneous LAA closure using an endocardial approach is based on delivery of the device via a percutaneous catheter introduced through a femoral vein that reaches the LAA via septal puncture. Implantation is guided by fluoroscopy and/or TEE. An intravenous heparin bolus is administered to achieve partial activated thromboplastin time of ≥250 s.20,27,28
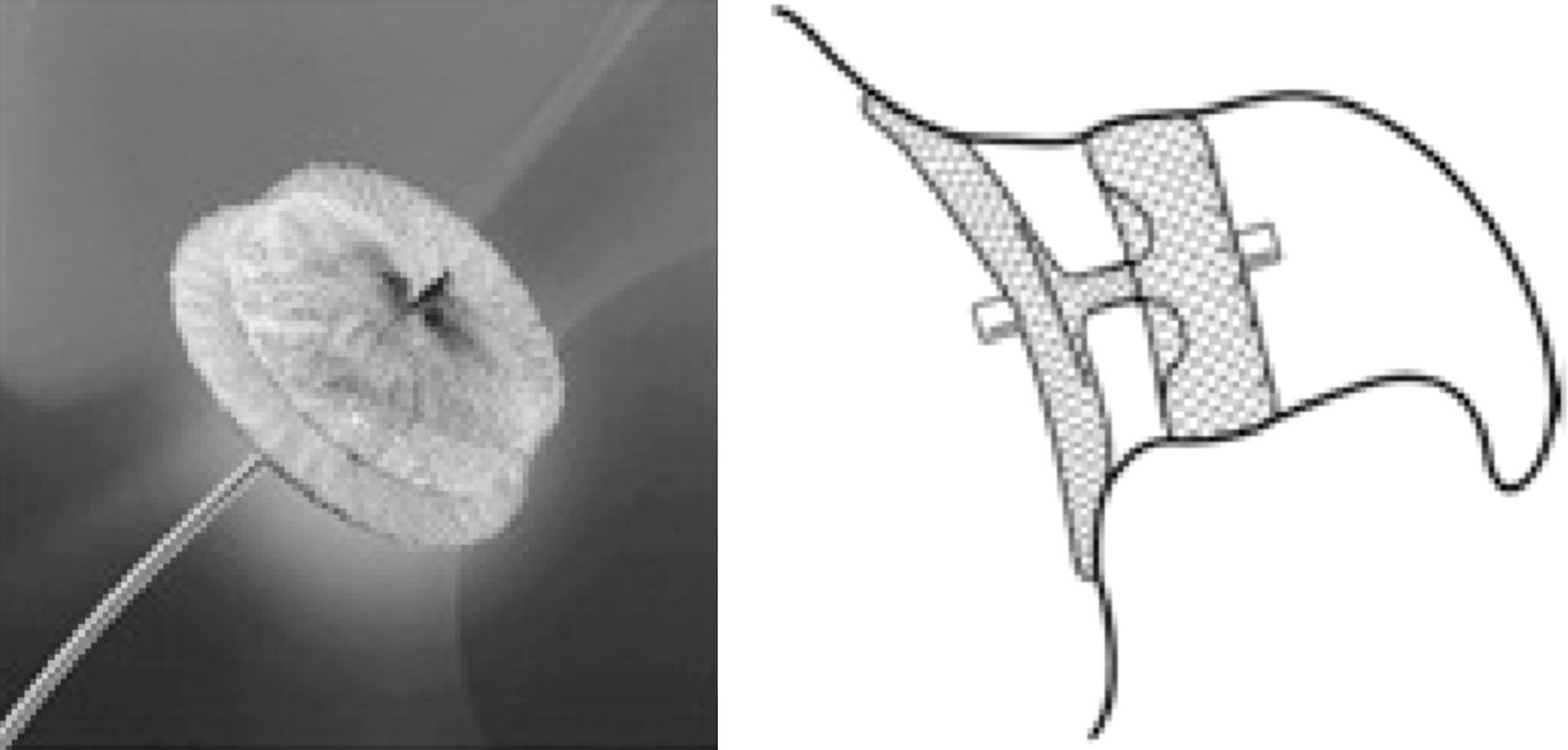
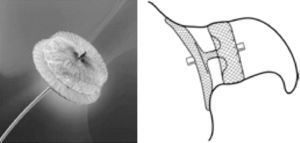
The PLAATO deviceThe first percutaneous LAA closure device was the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) system (ev3 Inc., Plymouth, MN).27 It consisted of a self-expanding nitinol cage coated with a polytetrafluoroethylene membrane designed both to occlude LAA flow and to allow tissue incorporation into the device. There were also three rows of anchors to attach the device to the LAA orifice (Figure 1).27
The PLAATO device (ev3 Inc., Plymouth, MN). LA: left atrium; LAA: left atrial appendage. Adapted from27.
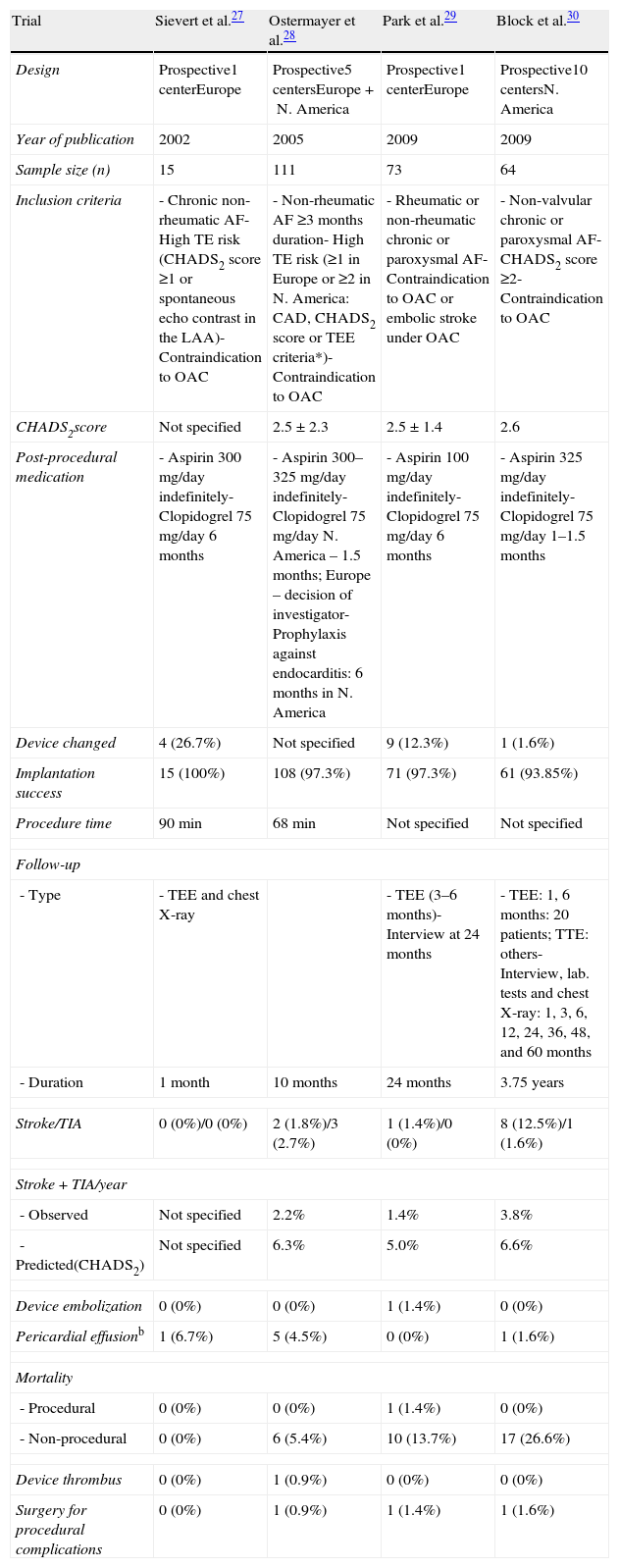
Table 3 lists data on the main clinical trials on the PLAATO device. The first clinical trial in Europe, by Sievert et al.,27 included 15 patients with chronic non-rheumatic AF, high risk for thromboembolism on CHADS2 criteria or with spontaneous echo contrast in the LAA and contraindications to warfarin. Device implantation was successful in all patients, with no residual shunt, new thrombus formation on the device, device migration or thromboembolic phenomena at one-month follow-up. The only complication was one case of pericardial effusion resolved by pericardiocentesis.27
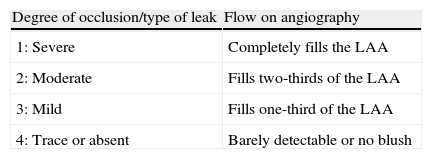
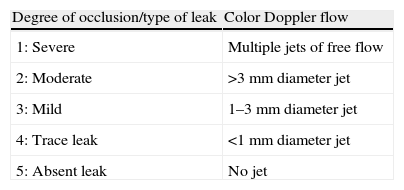
This was followed by an international multicenter prospective trial by Ostermayer et al.,28 which included results from five centers in Europe and North America and was the largest study on the PLAATO device. The study population consisted of patients with non-rheumatic AF of at least three months’ duration, contraindication to warfarin and high risk for thromboembolic events (history of myocardial infarction or significant coronary stenosis, CHADS2 criteria or moderate or dense spontaneous contrast or blood flow velocity ≤20cm/s within the LAA on echocardiography). It was the first study to use angiographic grading to evaluate LAA occlusion (Table 1), which was also assessed by transesophageal color Doppler echocardiography and graded on a five-point scale (Table 2).
Angiographic classification of left atrial appendage closure. Successful closure was defined by a leak of degree 3 or 4.
| Degree of occlusion/type of leak | Flow on angiography |
| 1: Severe | Completely fills the LAA |
| 2: Moderate | Fills two-thirds of the LAA |
| 3: Mild | Fills one-third of the LAA |
| 4: Trace or absent | Barely detectable or no blush |
LAA: left atrial appendage.
Classification of LAA closure by color Doppler echocardiography. Successful LAA occlusion was defined as a grade of 3 or higher.
| Degree of occlusion/type of leak | Color Doppler flow |
| 1: Severe | Multiple jets of free flow |
| 2: Moderate | >3mm diameter jet |
| 3: Mild | 1–3mm diameter jet |
| 4: Trace leak | <1mm diameter jet |
| 5: Absent leak | No jet |
Occlusion was successful in 97.3% of cases (108/111). During a mean follow-up of 10 months, the stroke rate was 2.2%; these two events occurred 6–7 months after the implant procedure, and in both cases the device was in stable position with no significant leak or adhering thrombi. The authors calculate that this rate was one-third of that predicted on the basis of the patients’ mean CHADS2 score of 2.5 points (estimated annual stroke rate of 6.3%).
Five patients among the first to undergo the technique experienced pericardial effusion, which was attributed to lack of experience with the technique. One required surgery and died from postoperative complications. There were no other significant complications and no instances of device dislocation or migration (Table 3).28
Characteristics and results of the main clinical trials on the PLAATO device (ev3, Inc., Plymouth, MN).
| Trial | Sievert et al.27 | Ostermayer et al.28 | Park et al.29 | Block et al.30 |
| Design | Prospective1 centerEurope | Prospective5 centersEurope+N. America | Prospective1 centerEurope | Prospective10 centersN. America |
| Year of publication | 2002 | 2005 | 2009 | 2009 |
| Sample size (n) | 15 | 111 | 73 | 64 |
| Inclusion criteria | - Chronic non-rheumatic AF- High TE risk (CHADS2 score ≥1 or spontaneous echo contrast in the LAA)- Contraindication to OAC | - Non-rheumatic AF ≥3 months duration- High TE risk (≥1 in Europe or ≥2 in N. America: CAD, CHADS2 score or TEE criteria*)- Contraindication to OAC | - Rheumatic or non-rheumatic chronic or paroxysmal AF- Contraindication to OAC or embolic stroke under OAC | - Non-valvular chronic or paroxysmal AF- CHADS2 score ≥2- Contraindication to OAC |
| CHADS2score | Not specified | 2.5±2.3 | 2.5±1.4 | 2.6 |
| Post-procedural medication | - Aspirin 300mg/day indefinitely- Clopidogrel 75mg/day 6 months | - Aspirin 300–325mg/day indefinitely- Clopidogrel 75mg/day N. America – 1.5 months; Europe – decision of investigator- Prophylaxis against endocarditis: 6 months in N. America | - Aspirin 100mg/day indefinitely- Clopidogrel 75mg/day 6 months | - Aspirin 325mg/day indefinitely- Clopidogrel 75mg/day 1–1.5 months |
| Device changed | 4 (26.7%) | Not specified | 9 (12.3%) | 1 (1.6%) |
| Implantation success | 15 (100%) | 108 (97.3%) | 71 (97.3%) | 61 (93.85%) |
| Procedure time | 90min | 68min | Not specified | Not specified |
| Follow-up | ||||
| - Type | - TEE and chest X-ray | - TEE (3–6 months)- Interview at 24 months | - TEE: 1, 6 months: 20 patients; TTE: others- Interview, lab. tests and chest X-ray: 1, 3, 6, 12, 24, 36, 48, and 60 months | |
| - Duration | 1 month | 10 months | 24 months | 3.75 years |
| Stroke/TIA | 0 (0%)/0 (0%) | 2 (1.8%)/3 (2.7%) | 1 (1.4%)/0 (0%) | 8 (12.5%)/1 (1.6%) |
| Stroke+TIA/year | ||||
| - Observed | Not specified | 2.2% | 1.4% | 3.8% |
| - Predicted(CHADS2) | Not specified | 6.3% | 5.0% | 6.6% |
| Device embolization | 0 (0%) | 0 (0%) | 1 (1.4%) | 0 (0%) |
| Pericardial effusionb | 1 (6.7%) | 5 (4.5%) | 0 (0%) | 1 (1.6%) |
| Mortality | ||||
| - Procedural | 0 (0%) | 0 (0%) | 1 (1.4%) | 0 (0%) |
| - Non-procedural | 0 (0%) | 6 (5.4%) | 10 (13.7%) | 17 (26.6%) |
| Device thrombus | 0 (0%) | 1 (0.9%) | 0 (0%) | 0 (0%) |
| Surgery for procedural complications | 0 (0%) | 1 (0.9%) | 1 (1.4%) | 1 (1.6%) |
AF: atrial fibrillation; CAD: coronary artery disease; LAA: left atrial appendage; OAC: oral anticoagulation; TEE: transesophageal echocardiography; TIA; transient ischemic attack; TTE: transthoracic echocardiography.
aTTE criteria – flow velocity in the LAA <20cm/s or moderate or dense spontaneous echocardiographic contrast.
bRequiring treatment (pericardiocentesis or surgery).
The largest series of patients treated with the PLAATO device, reported by Park et al.,29 was in a single German center. Percutaneous LAA closure was performed in 71 patients with rheumatic and non-rheumatic AF, most chronic but paroxysmal in eight cases, with contraindication to oral anticoagulation or history of stroke under anticoagulant therapy.
Occlusion was successful in 97.3% of patients. In a mean follow-up of 24 months, one minor stroke was reported (1.4%), a lower rate than the 5% predicted on the basis of a mean CHADS2 score of 2.5. One device embolization occurred, occluding the left ventricular outflow tract, resulting in the patient's death, and there was one case of device instability requiring removal by open surgery.
Ten deaths (7%) occurred during follow-up, but only one (described above) was procedure-related. There was one case of pericardial effusion, which resolved without pericardiocentesis (Table 3).29
A prospective non-randomized multicenter clinical trial in North America, that of Block et al.,30 analyzed 64 patients with non-valvular FA (chronic or paroxysmal) not eligible for chronic warfarin therapy and with CHADS2 score of ≥2. The procedure was successful in 93.8% of patients; mean follow-up was 3.75 years, and up to five years in some cases. The stroke rate was 3.8%: five major (between 7 and 53 months after the procedure) and three minor (8–23 months). There was one transient ischemic attack (TIA). The total stroke/TIA rate of 3.8% was little more than half that predicted by the mean CHADS2 score of 2.6 (6.6%).
There were two deaths, one from cerebral hemorrhage and the other from surgical complications unrelated to the device or the closure technique. The only procedure-related complication was a pericardial effusion requiring surgery (Table 3).30
Other smaller series have been published from different centers, all of them confirming the efficacy of the PLAATO device for preventing stroke in AF. However, the device was eventually withdrawn from the market by the manufacturer.
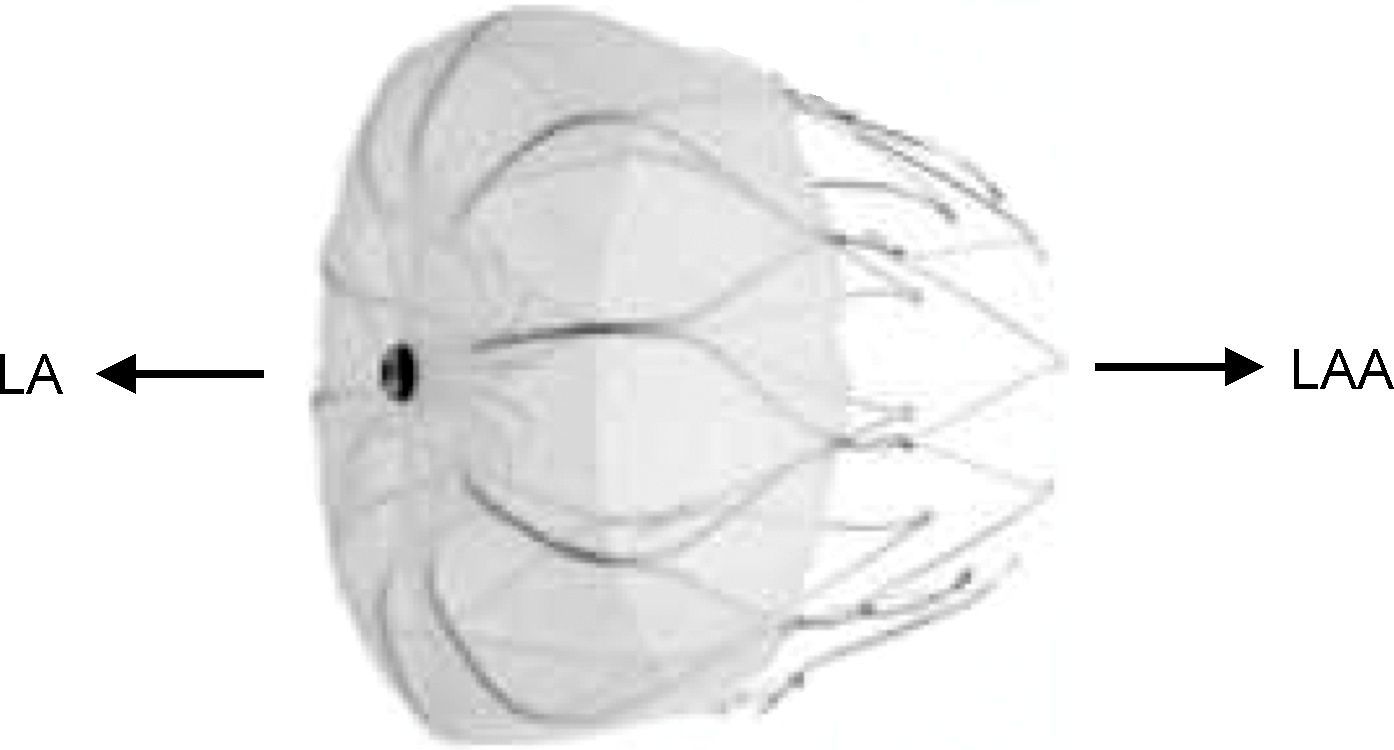
Amplatzer devicesThe second device to be used for percutaneous LAA closure was the Amplatzer septal occluder (St. Jude Medical, Plymouth, MN), which was originally used to close atrial septal defects (ASD). It was tested in 16 patients in four centers in 2002 by Meier et al.,31 who reported a single complication, embolization of an inappropriately sized device. The septal occluder was not used again for this purpose; instead, a device was developed specifically for LAA closure, the Amplatzer Cardiac Plug (ACP) (St. Jude Medical, Plymouth, MN),32 with a self-expanding nitilol frame covered in a polyester patch, consisting of a lobe and a disc connected by a central waist. Hooks on the lobe fix the device, while the disc seals the LAA orifice (Figure 2). It is available in eight lobe sizes from 16 to 30 mm at 2-mm intervals.32
The Amplatzer Cardiac Plug (St. Jude Medical, Plymouth, MN). Adapted from32.
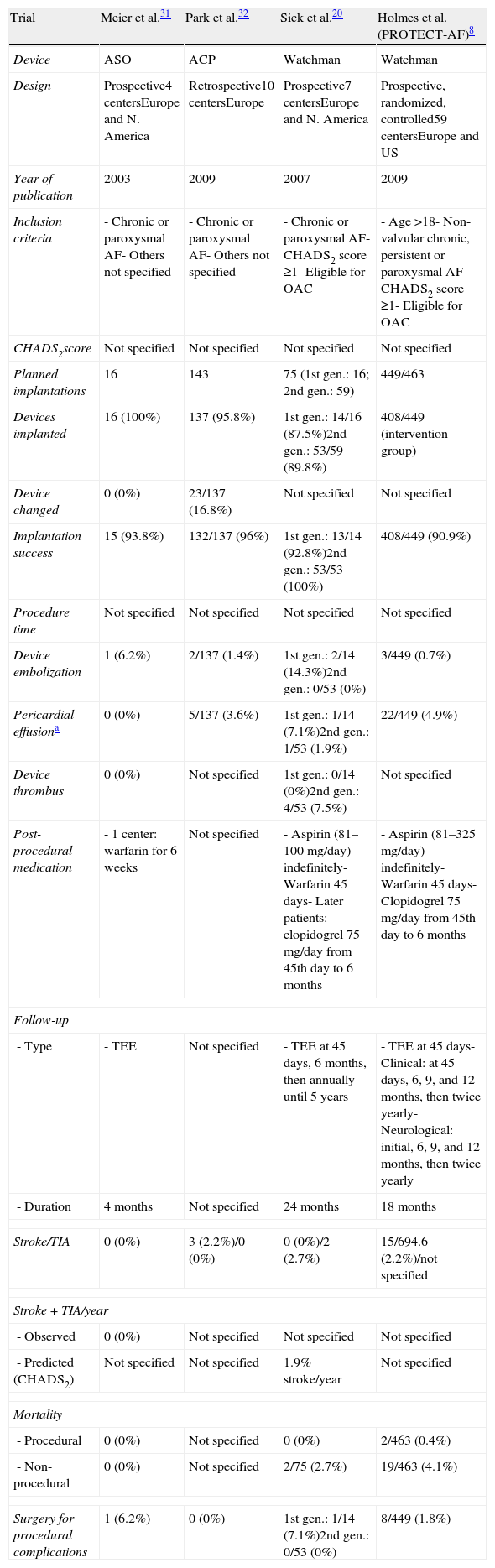
As the ACP began to be widely used, and in the light of safety concerns, Park et al.32 reviewed the experience of various centers to assess its safety profile, retrospectively analyzing implantations from its first use in December 2008 to November 2009, in a total of 143 AF patients in 10 European centers. Warfarin was suspended in all cases before ACP implantation, while dual antiplatelet therapy was maintained for 1–3 months, followed by single antiplatelet therapy indefinitely (Table 4).
Characteristics and results of the main clinical trials on the Amplatzer (St. Jude Medical, Plymouth, MN) and Watchman (Boston Scientific, Plymouth, MN) devices.
| Trial | Meier et al.31 | Park et al.32 | Sick et al.20 | Holmes et al. (PROTECT-AF)8 |
| Device | ASO | ACP | Watchman | Watchman |
| Design | Prospective4 centersEurope and N. America | Retrospective10 centersEurope | Prospective7 centersEurope and N. America | Prospective, randomized, controlled59 centersEurope and US |
| Year of publication | 2003 | 2009 | 2007 | 2009 |
| Inclusion criteria | - Chronic or paroxysmal AF- Others not specified | - Chronic or paroxysmal AF- Others not specified | - Chronic or paroxysmal AF- CHADS2 score ≥1- Eligible for OAC | - Age >18- Non-valvular chronic, persistent or paroxysmal AF- CHADS2 score ≥1- Eligible for OAC |
| CHADS2score | Not specified | Not specified | Not specified | Not specified |
| Planned implantations | 16 | 143 | 75 (1st gen.: 16; 2nd gen.: 59) | 449/463 |
| Devices implanted | 16 (100%) | 137 (95.8%) | 1st gen.: 14/16 (87.5%)2nd gen.: 53/59 (89.8%) | 408/449 (intervention group) |
| Device changed | 0 (0%) | 23/137 (16.8%) | Not specified | Not specified |
| Implantation success | 15 (93.8%) | 132/137 (96%) | 1st gen.: 13/14 (92.8%)2nd gen.: 53/53 (100%) | 408/449 (90.9%) |
| Procedure time | Not specified | Not specified | Not specified | Not specified |
| Device embolization | 1 (6.2%) | 2/137 (1.4%) | 1st gen.: 2/14 (14.3%)2nd gen.: 0/53 (0%) | 3/449 (0.7%) |
| Pericardial effusiona | 0 (0%) | 5/137 (3.6%) | 1st gen.: 1/14 (7.1%)2nd gen.: 1/53 (1.9%) | 22/449 (4.9%) |
| Device thrombus | 0 (0%) | Not specified | 1st gen.: 0/14 (0%)2nd gen.: 4/53 (7.5%) | Not specified |
| Post-procedural medication | - 1 center: warfarin for 6 weeks | Not specified | - Aspirin (81–100mg/day) indefinitely- Warfarin 45 days- Later patients: clopidogrel 75mg/day from 45th day to 6 months | - Aspirin (81–325mg/day) indefinitely- Warfarin 45 days- Clopidogrel 75mg/day from 45th day to 6 months |
| Follow-up | ||||
| - Type | - TEE | Not specified | - TEE at 45 days, 6 months, then annually until 5 years | - TEE at 45 days- Clinical: at 45 days, 6, 9, and 12 months, then twice yearly- Neurological: initial, 6, 9, and 12 months, then twice yearly |
| - Duration | 4 months | Not specified | 24 months | 18 months |
| Stroke/TIA | 0 (0%) | 3 (2.2%)/0 (0%) | 0 (0%)/2 (2.7%) | 15/694.6 (2.2%)/not specified |
| Stroke+TIA/year | ||||
| - Observed | 0 (0%) | Not specified | Not specified | Not specified |
| - Predicted (CHADS2) | Not specified | Not specified | 1.9% stroke/year | Not specified |
| Mortality | ||||
| - Procedural | 0 (0%) | Not specified | 0 (0%) | 2/463 (0.4%) |
| - Non-procedural | 0 (0%) | Not specified | 2/75 (2.7%) | 19/463 (4.1%) |
| Surgery for procedural complications | 1 (6.2%) | 0 (0%) | 1st gen.: 1/14 (7.1%)2nd gen.: 0/53 (0%) | 8/449 (1.8%) |
Requiring treatment (pericardiocentesis or surgery). 1st gen.: first-generation device; 2nd gen.: second-generation device; ACP: Amplatzer Cardiac Plug; AF: atrial fibrillation; ASO: Amplatzer septal occulder; OAC: oral anticoagulation; TEE: transesophageal echocardiography; TIA: transient ischemic attack.
The registry did not aim to assess indications for LAA closure or the effectiveness of the procedure, but to analyze the feasibility and safety of the ACP 24 hours after implantation. This was successful in 96% of patients; in the remainder implantation was unsuccessful due to device embolization (n=2) or unfavorable anatomy (n=3). The device needed replacing in 17% of cases.
Serious complications were seen in 10 patients (7%): three with stroke due to air embolism or intracardiac thrombus; device embolization in two cases, both percutaneously recaptured, with no sequelae; and five with pericardial effusion requiring pericardiocentesis. Minor complications were insignificant pericardial effusions in four, transient myocardial ischemia due to air embolism in two, and loss of the implant in the venous system in one patient, recovered percutaneously (Table 4).
The authors concluded that the ACP could be successfully implanted in a slightly higher proportion of patients than in the PROTECT AF trial,8 with a similar adverse event rate. They also stressed the importance of the learning curve in reducing procedure-related complications. The ability of the LAA to adapt to pressure was given as the main reason for incorrect device sizing, but the authors also pointed out that the ACP is a more flexible device and thus better able to adapt to the oval shape of the LAA orifice. One cause they identified for pericardial effusion was perforation of the LAA, due to its thin walls; as causes of periprocedural stroke, they reported air embolism and small LAA thrombi located in fully contracted lobes that were not visualized on TEE, and suggest these could be detected by multiple contrast injections. The periprocedural stroke rate was 2%.32
This registry made no mention of formation of thrombi on the ACP, but reports subsequently appeared of thrombi adhering to the device. In one case, a patient with chronic AF, CHADS2 score of 2 and contraindication to oral anticoagulation, underwent successful percutaneous LAA exclusion and was prescribed clopidogrel for one month and aspirin indefinitely, but TEE at three-month follow-up revealed a thrombus adhering to the device, which was resolved by enoxaparin 60mg twice daily and aspirin, continued thereafter.33 In another case, percutaneous LAA closure with the ACP was successfully performed in a patient with chronic AF, CHADS2 score of 6 and contraindication to oral anticoagulation due to chronic hematuria, who was prescribed clopidogrel and aspirin. Six-month follow-up TEE detected a thrombus on the device, treated by intravenous heparin, which took three weeks to have any effect in reducing the thrombus. Oral anticoagulation was accordingly started but had to be discontinued due to hematuria; no thromboembolic events were recorded.34
In view of increasing concerns over this complication, in January 2011 the manufacturer (at that time AGA Medical), after investigating these cases, published a Field Safety Notice for all centers implanting the ACP, updating the instructions for use, stating that the most likely cause for thrombus formation on the device was excessively deep implantation, and emphasizing the importance of measuring the depth of the LAA and width of the orifice at the implantation site. It also claims that adequacy of the anticoagulation and/or antiplatelet regimen can also be considered a potential contributor in thrombus formation, recommending aspirin for six months post-implant, leaving the decision to continue this regimen after six months at the discretion of the physician, and recommending clopidogrel or an alternate antiplatelet, with prescription following routine standard of care.35
In April 2011 Plicht et al. published a series assessing thrombus formation on the ACP in 31 patients, all of whom were medicated after implantation in accordance with the manufacturer's instructions. They underwent TEE before discharge and at three and six months; device thrombus was detected in three patients on the pre-discharge TEE and in three more at three months. In three of these patients the thrombi resolved after intravenous heparin administration for one week and oral anticoagulation was reinitiated for another three months in the other three, and one patient still had thrombus at the time of publication. The authors found no significant difference in the implantation site between those with and those without thrombi, and considered that thrombus formation in their series did not appear to be related to deep device implantation.36
The experience of a single Swiss center was published in May 2012 by Guérios et al.,37 assessing the safety and efficacy of the ACP in patients with non-valvular AF (chronic or paroxysmal) with at least one additional risk factor for thromboembolism and contradiction or intolerance to chronic oral anticoagulation. The procedure was guided by angiography alone. Patients were prescribed clopidogrel for one month and aspirin for 3–4 months, or lifelong if there was significant coronary artery disease. TEE was performed before discharge and 3–6 months after implantation. Procedural success was obtained in 85 of the 86 treated patients (99%). The unsuccessful case was attributed to a patent foramen ovale (PFO) previously closed with an Amplatzer septal occluder; repeated attempts at implantation resulted in pericardial tamponade, resolved by pericardial drainage. Eighty-seven devices were implanted in the other 85 patients; in 81 of them success was achieved with the first device chosen, while in four the device had to be changed. In two patients LAA closure was incomplete; in one of them, an additional ACP was used, and an Amplatzer vascular plug in the other, with good final results.
In 48 patients (55.8%) LAA closure was combined with another intervention, such as ASD closure, percutaneous coronary intervention, or transcatheter aortic valve implantation.
One ACP embolization occurred about 15 minutes after being released during percutaneous coronary angioplasty; the device was retrieved and replaced by a smaller ACP. During the procedure, there was a pericardial effusion in one patient with no hemodynamic compromise, two ischemic cerebral events, one due to air embolism and the other probably thromboembolic, and one death six days after implantation due to bleeding from a gastrointestinal tumor. Follow-up was obtained in 69 of the remaining patients (a total of 25.9 patient-years), during which there were two deaths, one non-cardiovascular (bronchopneumonia) and the other cardiovascular, in a patient with three-vessel coronary disease. No other embolic events or peri-device leaks were observed, but a non-mobile thrombus was detected in six patients, which disappeared after oral anticoagulation for three months. In four patients, the presence of a fixed thrombus could not be ruled out; in one warfarin was prescribed for four months, no change being observed in the control TEE, and the other three remained on aspirin. After reviewing the results, the authors concluded that in 70% of cases with thrombi the device had been implanted more deeply than was desirable.
The prospective randomized Amplatzer Cardiac Plug Clinical Trial is currently under way, comparing the efficacy of the ACP with warfarin in patients with AF and CHADS2 score ≥2, and without contraindication for oral coagulation.38
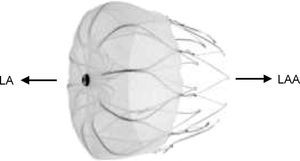
The Watchman left atrial appendage systemThe Watchman device (Boston Scientific, Plymouth, MN) was designed specifically for percutaneous LAA closure. It consists of a self-expanding nitinol frame with fixation barbs around its perimeter and a porous polyester membrane only on the LA-facing surface (Figure 3). It is available in diameters of 21, 24, 27, 30 and 33mm, which should be 10–20% greater than the LAA orifice.20
The Watchman device (Boston Scientific, Plymouth, MN). Adapted from9.
The first clinical trial, by Sick et al., aimed to assess the feasibility of this device for LAA closure.20 It prospectively included 75 patients from centers in Europe and the US with a history of chronic or paroxysmal AF and CHADS2 score ≥1 without contradiction for oral anticoagulation.
Following the procedure patients were medicated with aspirin (81–100mg daily), continued indefinitely, and warfarin for 45 days. Control TEE was performed at 45 days; if the LAA was successfully sealed according to the criteria of absence of flow or minimal flow around the device (jet of <3mm), warfarin was discontinued. The therapeutic regimen was later modified to include concomitant therapy with aspirin and 75mg clopidogrel between 45 days and the six-month follow-up.
Closure was successful in 93% of cases (54 of 58). The first 16 patients received a first-generation device, which was associated with a significant number of complications including two device embolizations, one air embolism leading to a malignant arrhythmia requiring cardiopulmonary resuscitation, and one fractured delivery wire. The delivery system was subsequently modified and the device was redesigned; the second-generation device was used in 53 patients and no further embolizations occurred. Pericardial effusions occurred in two of the 75 cases (2.6%) related to the transseptal puncture procedure, one to an overly vigorous “tug test” usually performed for proof of stability of the device. The technique was modified to observe the LAA during the tug, either by fluoroscopy or TEE, and no further tug-related effusions were observed.
No strokes occurred during the 24-month follow-up, compared with the expected rate of 1.9/year based on the CHADS2 score in this study cohort. Two cases of TIA occurred, one at four months without visible device thrombus and the other at six months with a smooth layer of thrombus detected on the surface of the device. Three more patients showed thrombus formation on the device surface without neurological symptoms. The authors concluded that the endothelialization process may not be completed at 45 days after implantation when warfarin is discontinued, so they modified the therapeutic regimen to include concomitant therapy with aspirin and clopidogrel between 45 days and the six-month follow-up.
There were three major bleeding complications, two of which were pericardial effusions requiring pericardiocentesis, and one internal bleed due to retrieval after device embolization. There were minor bleeds in two patients.
Two patients died during follow-up: one from dissection of the ascending aorta and the other from multiple organ failure after bowel surgery.
The authors considered the results comparable to those reported for the PLAATO system, but with early results potentially biased by the use of a first-generation device and operator learning curves.
The PROTECT AF trial,8,9 the first randomized clinical trial to directly compare percutaneous LAA closure (with the Watchman device) and warfarin, ran from February 2007 to June 2008, aiming to assess the non-inferiority of LAA closure in terms of efficacy and safety.8
The study population consisted of 707 patients in 59 centers in Europe and the US with non-valvular paroxysmal, persistent or chronic AF, CHADS2 score of ≥1 and without contraindication to warfarin. They were randomly assigned in a 2:1 ratio to device implantation (463 patients; intervention group) or warfarin therapy (244; control group). Mean follow-up was 18 months.
In the control group, the target INR of 2.0–3.0 was assessed every two weeks in the first six months, followed by monthly assessment. INR remained within the therapeutic range in 66% of measurements. In the intervention group warfarin was continued for 45 days after implantation in order to promote endothelization of the device. TEE was repeated at 45 days to verify successful closure (defined as no peri-device flow or flow <5mm wide (<3±2mm). If confirmed, warfarin was replaced by dual antiplatelet therapy with clopidogrel 75mg and aspirin 81–325mg for six months, followed by aspirin indefinitely. Follow-up consultations were scheduled for 45 days, six, nine and 12 months, and thenceforth twice a year. Neurological assessment was performed initially, at 12 and 24 months, and whenever a neurological event occurred.
Implantation of the Watchman device was successful in 88% of patients (408/463) and in 91% of those in whom it was attempted (408/449). Warfarin was discontinued at 45 days in 86% of patients and at six months in 92%.
Efficacy was assessed by a composite endpoint of ischemic stroke, hemorrhagic stroke, cardiovascular or unexplained death and systemic embolism. This occurred at a rate of 3%/year in the intervention group vs. 4.9%/year in the control group (rate ratio 0.62, 95% confidence interval 0.35–1.25), and the probability of non-inferiority of the intervention was more than 99.9%.
The total number of strokes was higher in the control group, but the rate of ischemic stroke was higher in the intervention group: one before implantation, five during (due to air embolism), and nine after. Six ischemic strokes were observed in the control group. INR assessed at the time of the stroke was subtherapeutic in both groups.
Hemorrhagic stroke was more common in the control group (6); five were fatal, all of which occurred with therapeutic warfarin levels. There was one hemorrhagic stroke under warfarin therapy in the intervention group.
There were 21 deaths in the intervention group (4.5%), due to stroke (2; 0.4%), cardiovascular or unexplained (4; 0.9%), and non-cardiovascular (15; 3.2%). In the control group there were 18 deaths (7.4%), due to stroke (6; 2.4%), cardiovascular or unexplained (6; 2.4%), and non-cardiovascular (6; 2.4%). Cumulative mortality in the intervention group and control group was 3% vs. 3.1% at one year and 5.9% vs. 9.1% at two years, respectively.
The safety composite endpoint was major bleeding (requiring transfusion of at least two units of packed red blood cells or surgical intervention) and procedure-related complications including stroke, device embolization or severe pericardial effusion requiring percutaneous or surgical drainage. These events were more frequent in the intervention group (7.4%/year vs. 4.4%/year) and earlier (55% on the day of the procedure vs. 50% between 45 days and one year) than in the control group. The most frequent safety event in the intervention group was severe pericardial effusion, seen in 4.8% of patients (n=22), requiring surgery in seven cases but resolved by pericardiocentesis in the others, with no associated mortality. The frequency of this complication, which was more common in the first three patients (7.1%) than in the others (4.4%) in all centers, declined as operator experience increased.
There was device embolization in three patients; one was identified during the procedure and the device was successfully removed percutaneously, and two others were asymptomatic, only being detected by TEE after 45 days and removed surgically.
The authors concluded that in terms of efficacy, percutaneous closure was not inferior to warfarin, even when patients at lower thromboembolic risk (CHADS2 score 1) were excluded. With regard to safety, the most common complications became less frequent as operators gained experience with the procedure, and none led to permanent disability or death. They suggested that the initial risk associated with device implantation was less than the cumulative risk of chronic warfarin therapy.
The main limitation to applying these results to all AF patients was the inclusion of patients at low embolic risk (65% with CHADS2 score 1 or 2). It is not known to what extent they would be reproducible in a higher-risk population, particularly with contraindication for warfarin.
In view of these findings, the FDA recommended a longer follow-up period and an ongoing registry was established to assess the safety of LAA closure with the Watchman device – the Continued Access Protocol (CAP) Registry.39
This registry, published in January 2011, included the 542 patients who underwent attempted LAA device closure in the intervention group of the PROTECT AF trial and a further 460 patients from 26 centers that participated in the trial with the same inclusion criteria, follow-up and medication. The authors emphasized the importance of flushing the sheath with saline to minimize the chance of air embolism.
Median follow-up was 2.5 years for PROTECT AF (0–4.7 years) and 0.4 years for CAP (0–1.6 years).
The efficacy composite endpoint was similar to that of PROTECT AF. Pericardial effusions were considered serious if they extended hospitalization.
The populations of the two studies differed mainly in age and CHADS2 score, which were higher in CAP (mean age 74±8 vs. 72±9 and CHADS2 score 2.4±1.2 vs. 2.2±1.2, p<0.001 for both).
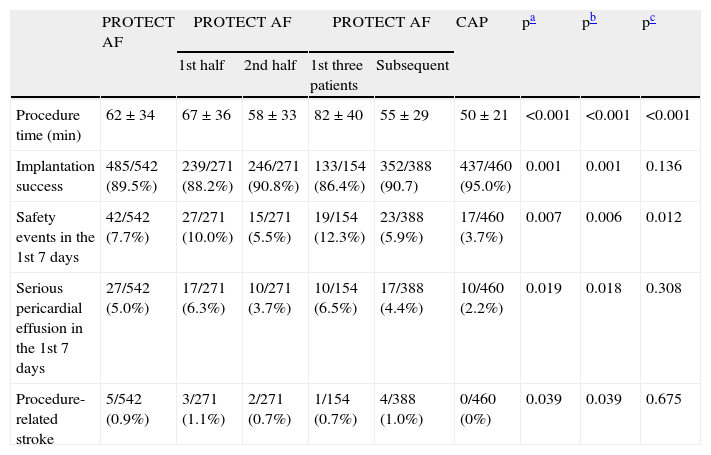
To assess the effect of the learning curve and operator experience, the results of CAP and PROTECT AF were compared, and the first half of the PROTECT AF patient cohort was compared with the second half of the cohort, as well as the first three patients enrolled at each site in PROTECT AF with all subsequent patients enrolled at that site. There was a reduction in mean procedure time, an increase in implantation success, and a decline in the number of safety events, serious pericardial effusions and procedural stroke between the first and second halves of PROTECT AF and between the second half of PROTECT AF and CAP (Table 5), the event rate in the second half of PROTECT AF (5.5%) being close to that seen in CAP (3.7%). Comparing the first three patients with all subsequent patients at each site, mean procedure time improved by 33% and the safety event rate improved by 52% (Table 5).
Safety events in PROTECT AF and CAP, adapted from39. Results expressed in n/total (%); p values obtained by the chi-square test or ANOVA.
| PROTECT AF | PROTECT AF | PROTECT AF | CAP | pa | pb | pc | |||
| 1st half | 2nd half | 1st three patients | Subsequent | ||||||
| Procedure time (min) | 62±34 | 67±36 | 58±33 | 82±40 | 55±29 | 50±21 | <0.001 | <0.001 | <0.001 |
| Implantation success | 485/542 (89.5%) | 239/271 (88.2%) | 246/271 (90.8%) | 133/154 (86.4%) | 352/388 (90.7) | 437/460 (95.0%) | 0.001 | 0.001 | 0.136 |
| Safety events in the 1st 7 days | 42/542 (7.7%) | 27/271 (10.0%) | 15/271 (5.5%) | 19/154 (12.3%) | 23/388 (5.9%) | 17/460 (3.7%) | 0.007 | 0.006 | 0.012 |
| Serious pericardial effusion in the 1st 7 days | 27/542 (5.0%) | 17/271 (6.3%) | 10/271 (3.7%) | 10/154 (6.5%) | 17/388 (4.4%) | 10/460 (2.2%) | 0.019 | 0.018 | 0.308 |
| Procedure-related stroke | 5/542 (0.9%) | 3/271 (1.1%) | 2/271 (0.7%) | 1/154 (0.7%) | 4/388 (1.0%) | 0/460 (0%) | 0.039 | 0.039 | 0.675 |
The time dependence of safety events was assessed by separating those that occurred within seven days of the procedure from those in the remainder of follow-up; overall, 94% of these events occurred in the first seven days (91% in PROTECT AF and 100% in CAP). Serious pericardial effusion was observed in 3.8% of patients (5.2% in PROTECT AF and 2.2% in CAP). The procedure-related stroke rate was 0.9% in PROTECT AF and zero in CAP. In PROTECT AF, device embolization occurred in 0.6% of patients vs. 0% in CAP. Device-associated thrombus was observed in 4.2% (20) of patients in PROTECT AF and in none in CAP.
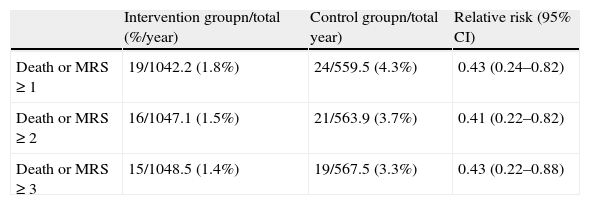
Another important analysis of the PROTECT AF results was to determine the functional impact of adverse events, identifying those that resulted in death or significant disability (defined as an increase in the modified Rankin score). This analysis revealed that regardless of how “significant” is defined (change in Rankin score of ≥1, ≥2 or ≥3), the safety event rate with functional impact was lower in the intervention group than in the control group, with a relative risk of ≈0.40 (Table 6).
Functional impact of safety events in PROTECT AF.
| Intervention groupn/total (%/year) | Control groupn/total year) | Relative risk (95% CI) | |
| Death or MRS≥1 | 19/1042.2 (1.8%) | 24/559.5 (4.3%) | 0.43 (0.24–0.82) |
| Death or MRS≥2 | 16/1047.1 (1.5%) | 21/563.9 (3.7%) | 0.41 (0.22–0.82) |
| Death or MRS≥3 | 15/1048.5 (1.4%) | 19/567.5 (3.3%) | 0.43 (0.22–0.88) |
CI: confidence interval; MRS: modified Rankin score.
The authors concluded that safety events in the Watchman group were largely procedure-related, that these safety events decreased in frequency with greater operator experience, and that they led to less significant disability than those related to warfarin therapy, which accumulate linearly over time.39
The protocols of both PROTECT AF and the CAP registry included a 45-day period of warfarin medication after percutaneous LAA closure, which limits their application to patients with contraindication to oral anticoagulation, for whom this procedure could be an alternative. The ASA Plavix Feasibility Study With WATCHMAN Left Atrial Appendage Closure Technology (ASAP),40 a prospective non-randomized registry, was set up to assess the efficacy of percutaneous LAA closure in these patients. It has the same inclusion and exclusion criteria as PROTECT AF, except for contraindication to warfarin, and includes 150 patients with non-valvular AF in three centers in Germany and one in the Czech Republic who underwent percutaneous closure with the Watchman device. After the procedure patients took clopidogrel for six months and aspirin indefinitely, and underwent TEE at three and 12 months. Preliminary results were presented at the Heart Rhythm Society 2012 Scientific Sessions.41 Implantation was successful in 94% of patients (141/150). In a follow-up of 14.2±8.7 months there was one systemic embolization, six cases of device-related thrombus and four strokes, an ischemic stroke rate of 1.8%, which according to the authors corresponds to a 75% reduction in events compared to that expected on the basis of a mean CHADS2 score of 2.8±1.2 if they had been taking aspirin alone (7.1%) and a 64% reduction compared to that expected under treatment with aspirin and clopidogrel indefinitely (5%). The authors concluded that implantation of the Watchman device was safe and effective without temporary warfarin therapy in patients with contraindication to oral anticoagulation.41
Incomplete closure of the LAA by surgical methods increases risk for embolic events, but it was not known whether this was also true of incomplete percutaneous closure. Viles-Gonzalez et al. accordingly performed a retrospective analysis of the intervention group of PROTECT AF, published in March 2012.42 They assessed peri-device blood flow, classified as minor, moderate, or major (<1mm, 1–3mm, and >3mm, respectively), by TEE at 45 days, six and 12 months. Of the 485 patients with a successfully implanted Watchman device, only 445 underwent TEE at 45 days, 414 at six months and 389 at 12 months. The prevalence of any flow around the device decreased with time from 40.9% at the 45-day TEE, to 33.8% at six months, and to 32.1% at 12 months (p=0.001). The severity of the flow at 45 days was minor in 7.7%, moderate in 59.9% and major in 32.4%; this distribution did not change significantly at six or 12 months (p=0.731). The mean and maximum width of the leak were 2.8 and 6.2mm, 2.9 and 6.8mm, and 2.9 and 6.0mm, at 45 days, six months, and 12 months, respectively.
There were no significant differences in the efficacy endpoint of PROTECT AF between patients with peri-device flow (2.8%) and those without (2%; p=0.635), or between those with any flow and no flow (hazard ratio [HR] 0.85, 0.83 and 0.48 for minor, moderate and major flow, respectively, p=0.798). The impact of 1-mm increases in flow size on this endpoint was also analyzed and there was no significant difference between the groups (HR 0.84, p=0.256).
Analysis of a second endpoint of stroke and systemic embolization again showed no significant relationship with the presence or severity of peri-device flow.
This analysis was also performed separately for patients who continued warfarin therapy after 45 days and those who discontinued it at that point, once again without showing any difference regarding the presence of peri-device flow (p=0.857).
The authors concluded that in this sample there was no relationship between the presence of residual peri-device flow and embolic events, although with the caveat that these results may not be applicable to devices other than the Watchman, and suggest that different devices should be analyzed in the same way.42
A second randomized clinical trial on the Watchman device is under way, the PREVAIL study.43 This has a similar structure to PROTECT AF, except that patients with CHADS2 score of 1 are only included if any of the following apply: female age 75 or older; left ventricular ejection fraction ≥30 and <35%, aged 65–74 and has diabetes or coronary artery disease, or aged ≥65 and has congestive heart failure.
ConclusionsThe development of percutaneous LAA closure was an important step in the prevention of cardioembolic events in AF, particularly in non-valvular AF. The feasibility of the technique has been demonstrated in various studies, the most important of which, PROTECT AF, showed similar efficacy to warfarin in prevention of thromboembolic events, although with a higher rate of safety events, particularly procedure-related. Longer follow-up of these patients and the establishment of the CAP registry have produced encouraging results for continued use of the technique, since they show that procedure-related complications decline with greater operator experience and lead to lower mortality and disability than warfarin therapy, suggesting that LAA closure has a better long-term safety profile.
These findings demonstrate that percutaneous LAA closure is a valid alternative for thromboembolic prevention in high-risk patients with non-valvular AF without contraindication for oral anticoagulation, although it has mainly been used in patients in whom anticoagulation is contraindicated, for obvious reasons. However, reports of a particularly serious complication – the formation of thrombi on the device – have cooled the initial enthusiasm generated by the technique and raised the possibility that a period of anticoagulation may be necessary after implantation in some patients.
Unlike the data on incomplete surgical LAA occlusion, incomplete closure with the Watchman device was not associated with embolic events in the only trial performed to date.
Although further studies are needed to assess possible late complications related to these devices and to determine any physiological consequences of LAA closure (about which little is known), percutaneous LAA closure appears to be a safe and effective alternative to oral anticoagulation in high-risk patients, and may also become a valid alternative for low-risk patients. The role of new oral anticoagulants in thromboembolic prevention will also be important in determining which patients would benefit from percutaneous LAA closure.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Faustino A, et al. Encerramento percutâneo do apêndice auricular esquerdo para profilaxia de tromboembolismo na fibrilhação auricular. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.06.017.