The impact of digoxin on outcomes of patients with advanced heart failure (HF) remains uncertain and its effect may be different for patients in atrial fibrillation (AF) or sinus rhythm (SR).
ObjectivesTo determine the impact of digoxin on outcomes of advanced HF patients and to assess whether prognosis differs in patients in AF and SR.
MethodsA total of 268 consecutive patients admitted to an intensive care unit with decompensated HF were evaluated. Patients were divided into two groups: A – patients with AF (n=89), and B – patients in SR (n=179). For each group we compared patients medicated and not medicated with digoxin. A mean follow-up of 3.3 years was performed.
ResultsAddition of digoxin to contemporary standard HF therapy showed no impact on mortality of patients in group B (all-cause mortality in follow-up: 19.1% vs. 22.5%, p=0.788). Regarding group A, we observed significantly lower medium-term mortality for patients on digoxin therapy (18.6% vs. 46.6%, p=0.048). Digoxin therapy did not influence readmissions for decompensated HF. Among AF patients, no differences were found regarding demographic, clinical, echocardiographic and laboratory variables between patients medicated and not medicated with digoxin.
ConclusionsDigoxin therapy may improve the prognosis of advanced HF patients with AF under optimal medical therapy. However, no benefit of digoxin was demonstrated for patients in SR. These results may help to improve patient selection for digoxin therapy.
O impacto prognóstico da digoxina na insuficiência cardíaca (IC) avançada permanece mal esclarecido. A relevância da terapêutica digitálica pode ser diferente na fibrilhação auricular (FA) relativamente ao ritmo sinusal (RS).
ObjectivosDeterminar o impacto prognóstico da digoxina na IC e verificar se este é diferente consoante se encontrem em FA ou em RS.
MétodosEstudaram-se 268 doentes internados numa unidade de cuidados intensivos por IC descompensada. Dividiu-se a população em dois grupos: A – 89 doentes em FA; grupo B – 179 doentes em RS. Para cada grupo compararam-se os doentes medicados com os não medicados com digoxina. Realizou-se um seguimento clínico com a duração mediana de 3,3 anos.
ResultadosA digoxina não teve impacto na mortalidade dos doentes com IC avançada que se encontravam em RS (mortalidade a 3,3 anos: 19,1% versus 22,5%, p=0,788), adicionada à terapêutica médica otimizada. Nos doentes em FA observou-se uma redução significativa da mortalidade nos doentes medicados com digoxina (18,6% versus 46,6%, p=0,048). A digoxina não alterou a taxa de reinternamentos por IC descompensada. No grupo A não se verificaram diferenças entre os doentes medicados e não medicados com digoxina relativamente aos parâmetros demográficos, clínicos, ecocardiográficos ou laboratoriais.
ConclusãoEste estudo sugere que a digoxina pode melhorar o prognóstico dos doentes com IC avançada e FA, sob terapêutica médica optimizada. Contudo, esta não demonstrou benefício nos doentes em RS. Estes resultados podem contribuir para uma melhor seleção dos doentes a medicar com digoxina, otimizando a relação risco/benefício desta terapêutica.
Despite more than 200 years of research, the role of digoxin in heart failure therapy remains controversial. The American College of Cardiology and American Heart Association (ACC/AHA) guidelines for the management of patients with heart failure (HF) recommend the use of digoxin, unless contraindicated, for persistent symptoms after optimal medical therapy.1 Digoxin use is also endorsed by the European Society of Cardiology guidelines.2 Although widely accepted, these recommendations are derived mainly from short-term studies assessing non-mortality outcomes.3–8 The Digitalis Investigator Group (DIG) study,9 a large randomized trial, showed no impact on all-cause or cardiovascular mortality with digoxin in oligosymptomatic male HF patients in sinus rhythm (SR). However, a reduction in hospitalizations due to worsening HF and an improvement in quality of life were reported.10 Two other trials showed that withdrawal of digoxin resulted in worsening HF symptoms.6,7 Although more than 90% of patients were receiving angiotensin-converting enzyme inhibitors (ACEIs) in the DIG trial,9 the use of beta-blockers and aldosterone antagonists was not reported and likely was very limited. Similarly, improved outcomes are also seen with cardiac devices, which are now widely used in HF patients. A recently published study suggests no benefit of digoxin in advanced HF patients on contemporary medical therapy.11 In this report, HF patients in SR treated with digoxin had a worse prognosis than those who were not. However, this did not appear to be true for patients in atrial fibrillation (AF). In patients with AF, the beneficial role of digoxin is widely accepted, mainly to optimize ventricular rate control, and the Carvedilol Atrial Fibrillation Evaluation (CAFE) study suggests that there may be synergistic benefits between digoxin and carvedilol.11
In our study, we sought to determine the impact of digoxin therapy in advanced HF patients on optimal contemporary medical therapy, and to assess whether prognosis differs according to the presence of AF.
MethodsPatient population and data collectionThis single-center study included 268 consecutive patients admitted to an intensive care unit between January 2003 and June 2006 due to decompensated HF in New York Heart Association (NYHA) class III or IV. Standardized records were used to describe the study population in terms of clinical and demographic characteristics, cardiovascular risk factors and comorbidities. The records also showed HF etiology, hemodynamic status on admission, laboratory parameters, electrocardiographic and echocardiographic data, and treatment. Renal dysfunction was defined as creatinine clearance of <60ml/min calculated by the Cockcroft–Gault formula. Ischemic cardiomyopathy was defined as left ventricular dysfunction associated with significant coronary disease (>70% stenosis in at least one major epicardial coronary artery and/or >50% left main stenosis). In the minority of patients without coronary angiography (4%) the diagnostic criterion of ischemic heart disease was prior myocardial infarction (history of infarction or infarct scar on ECG). Non-ischemic cardiomyopathy was defined as left ventricular dysfunction in the absence of the above alterations. Coronary angiography was performed in 70% of this group; an ischemia stress test (stress echocardiography, myocardial perfusion scanning or perfusion magnetic resonance imaging) was available in the remainder, which revealed no significant alterations. A mean clinical follow-up of 40 months was performed. Clinical data were collected during patients’ visits to the outpatient clinic or by telephone interview. The occurrence of readmission due to decompensated HF and death was recorded. Medical records were reviewed to confirm and to obtain additional information. The population was divided into two groups, according to the presence of AF: group A – 89 patients with AF and group B – 179 patients in sinus rhythm. A previously defined subgroup analysis was performed in patients in SR vs. AF. For each group patients medicated and not medicated with digoxin were compared.
EndpointsThe primary endpoint was long-term all-cause mortality and the secondary endpoint was a combined outcome of mortality and rehospitalization due to decompensated HF.
Statistical analysisThe statistical analysis was performed with SPSS 13.0 for Windows (SPSS Inc., Chicago, Ill.). The results were expressed as means ± standard deviation for continuous variables and as frequencies and percentages for categorical variables. The frequencies of the categorical variables in the two groups were compared by the chi-square test or Fisher's exact test. Continuous variables were compared using the Student's unpaired t test (normal variables) and the Mann–Whitney U test (non-normal variables). Kaplan–Meier survival analysis and the Breslow test were used to compare the groups in terms of cumulative survival. A value of p<0.05 was considered statistically significant.
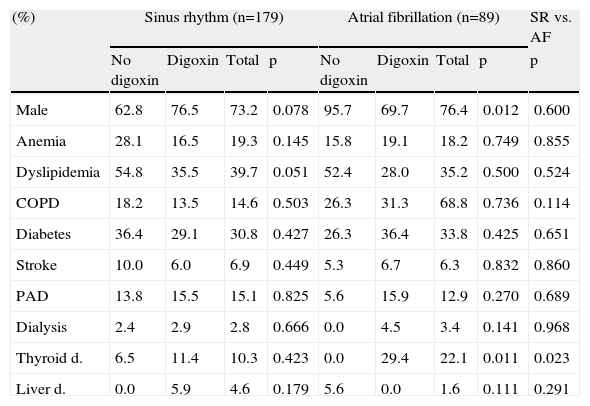
ResultsGeneral characteristics of the study groupsThe baseline characteristics of the study population are listed in Table 1. Of the total population hospitalized due to advanced HF during the study period, 33.2% (89/268) presented with AF. These patients were older (62.4±12.8 vs. 55.1±15.4 years; p<0.001), with higher left ventricular ejection fraction (LVEF) (31.0% vs. 26.0%, p=0.003) and a higher prevalence of valvular disease as HF etiology (3.4% vs. 13.6%; p<0.001). The prevalence of thyroid disease was higher in AF patients (22.1% vs. 10.3%; p=0.023).
General characteristics of the study population.
| (%) | Sinus rhythm (n=179) | Atrial fibrillation (n=89) | SR vs. AF | ||||||
| No digoxin | Digoxin | Total | p | No digoxin | Digoxin | Total | p | p | |
| Male | 62.8 | 76.5 | 73.2 | 0.078 | 95.7 | 69.7 | 76.4 | 0.012 | 0.600 |
| Anemia | 28.1 | 16.5 | 19.3 | 0.145 | 15.8 | 19.1 | 18.2 | 0.749 | 0.855 |
| Dyslipidemia | 54.8 | 35.5 | 39.7 | 0.051 | 52.4 | 28.0 | 35.2 | 0.500 | 0.524 |
| COPD | 18.2 | 13.5 | 14.6 | 0.503 | 26.3 | 31.3 | 68.8 | 0.736 | 0.114 |
| Diabetes | 36.4 | 29.1 | 30.8 | 0.427 | 26.3 | 36.4 | 33.8 | 0.425 | 0.651 |
| Stroke | 10.0 | 6.0 | 6.9 | 0.449 | 5.3 | 6.7 | 6.3 | 0.832 | 0.860 |
| PAD | 13.8 | 15.5 | 15.1 | 0.825 | 5.6 | 15.9 | 12.9 | 0.270 | 0.689 |
| Dialysis | 2.4 | 2.9 | 2.8 | 0.666 | 0.0 | 4.5 | 3.4 | 0.141 | 0.968 |
| Thyroid d. | 6.5 | 11.4 | 10.3 | 0.423 | 0.0 | 29.4 | 22.1 | 0.011 | 0.023 |
| Liver d. | 0.0 | 5.9 | 4.6 | 0.179 | 5.6 | 0.0 | 1.6 | 0.111 | 0.291 |
AF: atrial fibrillation; COPD: chronic obstructive pulmonary disease; d: disease; PAD: peripheral arterial disease; SR: sinus rhythm.
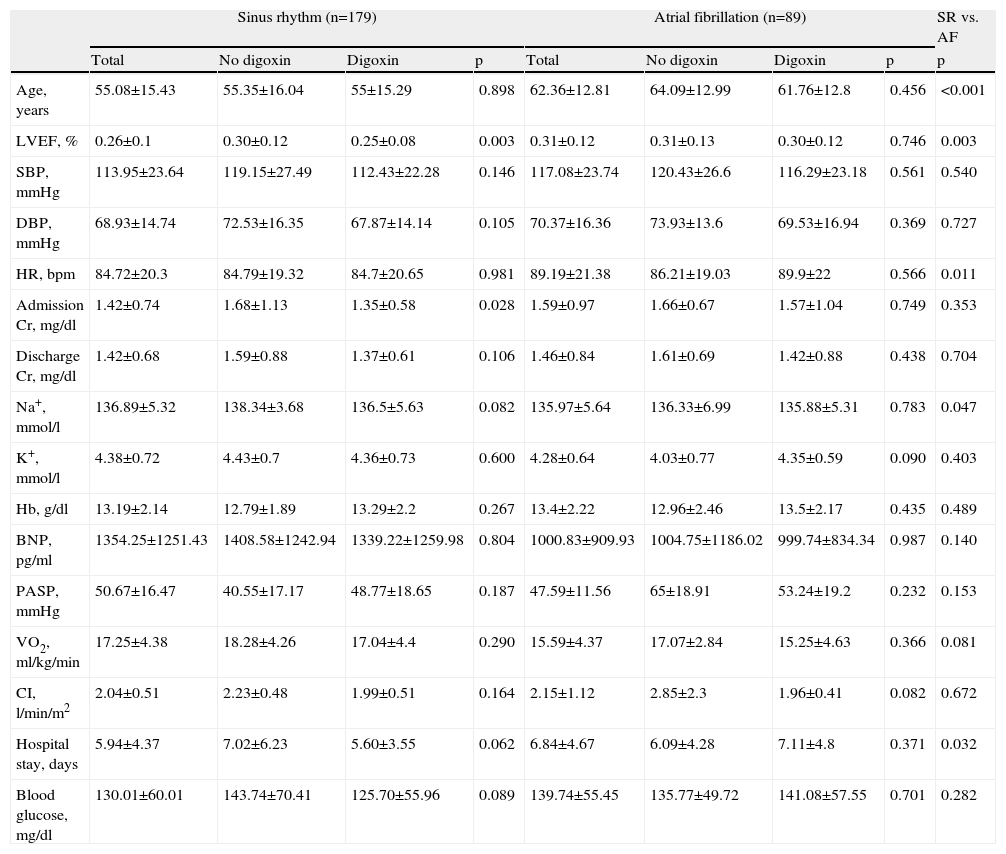
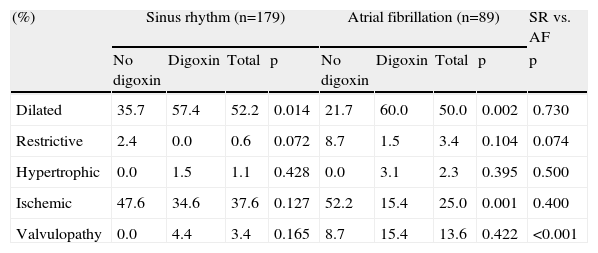
Among the AF patients, there were no significant differences between patients with or without digoxin regarding demographic parameters such as age or gender. We observed a higher prevalence of dyslipidemia in patients not medicated with digoxin (64.7% vs. 31.7%, p=0.039), but no differences were found between the groups regarding other cardiovascular risk factors or comorbidities. Analysis of hemodynamic, laboratory and echocardiographic data on admission showed no significant differences in blood pressure, heart rate, diuresis rate, LVEF, serum sodium, potassium, hemoglobin and B-type natriuretic peptide (BNP) levels, cardiac output, peak VO2 or pulmonary artery systolic pressure (Table 2). Dilated cardiomyopathy was more frequent in patients on digoxin therapy (86.8% vs. 13.2%, p=0.032) and ischemic cardiomyopathy was more prevalent in patients without this therapy (37.5% vs. 62.5%, p<0.001), as shown in Table 3.
Hemodynamic, echocardiographic and laboratory data.
| Sinus rhythm (n=179) | Atrial fibrillation (n=89) | SR vs. AF | |||||||
| Total | No digoxin | Digoxin | p | Total | No digoxin | Digoxin | p | p | |
| Age, years | 55.08±15.43 | 55.35±16.04 | 55±15.29 | 0.898 | 62.36±12.81 | 64.09±12.99 | 61.76±12.8 | 0.456 | <0.001 |
| LVEF, % | 0.26±0.1 | 0.30±0.12 | 0.25±0.08 | 0.003 | 0.31±0.12 | 0.31±0.13 | 0.30±0.12 | 0.746 | 0.003 |
| SBP, mmHg | 113.95±23.64 | 119.15±27.49 | 112.43±22.28 | 0.146 | 117.08±23.74 | 120.43±26.6 | 116.29±23.18 | 0.561 | 0.540 |
| DBP, mmHg | 68.93±14.74 | 72.53±16.35 | 67.87±14.14 | 0.105 | 70.37±16.36 | 73.93±13.6 | 69.53±16.94 | 0.369 | 0.727 |
| HR, bpm | 84.72±20.3 | 84.79±19.32 | 84.7±20.65 | 0.981 | 89.19±21.38 | 86.21±19.03 | 89.9±22 | 0.566 | 0.011 |
| Admission Cr, mg/dl | 1.42±0.74 | 1.68±1.13 | 1.35±0.58 | 0.028 | 1.59±0.97 | 1.66±0.67 | 1.57±1.04 | 0.749 | 0.353 |
| Discharge Cr, mg/dl | 1.42±0.68 | 1.59±0.88 | 1.37±0.61 | 0.106 | 1.46±0.84 | 1.61±0.69 | 1.42±0.88 | 0.438 | 0.704 |
| Na+, mmol/l | 136.89±5.32 | 138.34±3.68 | 136.5±5.63 | 0.082 | 135.97±5.64 | 136.33±6.99 | 135.88±5.31 | 0.783 | 0.047 |
| K+, mmol/l | 4.38±0.72 | 4.43±0.7 | 4.36±0.73 | 0.600 | 4.28±0.64 | 4.03±0.77 | 4.35±0.59 | 0.090 | 0.403 |
| Hb, g/dl | 13.19±2.14 | 12.79±1.89 | 13.29±2.2 | 0.267 | 13.4±2.22 | 12.96±2.46 | 13.5±2.17 | 0.435 | 0.489 |
| BNP, pg/ml | 1354.25±1251.43 | 1408.58±1242.94 | 1339.22±1259.98 | 0.804 | 1000.83±909.93 | 1004.75±1186.02 | 999.74±834.34 | 0.987 | 0.140 |
| PASP, mmHg | 50.67±16.47 | 40.55±17.17 | 48.77±18.65 | 0.187 | 47.59±11.56 | 65±18.91 | 53.24±19.2 | 0.232 | 0.153 |
| VO2, ml/kg/min | 17.25±4.38 | 18.28±4.26 | 17.04±4.4 | 0.290 | 15.59±4.37 | 17.07±2.84 | 15.25±4.63 | 0.366 | 0.081 |
| CI, l/min/m2 | 2.04±0.51 | 2.23±0.48 | 1.99±0.51 | 0.164 | 2.15±1.12 | 2.85±2.3 | 1.96±0.41 | 0.082 | 0.672 |
| Hospital stay, days | 5.94±4.37 | 7.02±6.23 | 5.60±3.55 | 0.062 | 6.84±4.67 | 6.09±4.28 | 7.11±4.8 | 0.371 | 0.032 |
| Blood glucose, mg/dl | 130.01±60.01 | 143.74±70.41 | 125.70±55.96 | 0.089 | 139.74±55.45 | 135.77±49.72 | 141.08±57.55 | 0.701 | 0.282 |
BNP: B-type natriuretic peptide; CI: cardiac index by the Fick method; Cr: creatinine; DBP: diastolic blood pressure; Hb: hemoglobin; HR: heart rate; LVEF: left ventricular ejection fraction; PASP: pulmonary artery systolic pressure; SBP: systolic blood pressure; VO2: peak oxygen uptake.
Etiology of heart failure.
| (%) | Sinus rhythm (n=179) | Atrial fibrillation (n=89) | SR vs. AF | ||||||
| No digoxin | Digoxin | Total | p | No digoxin | Digoxin | Total | p | p | |
| Dilated | 35.7 | 57.4 | 52.2 | 0.014 | 21.7 | 60.0 | 50.0 | 0.002 | 0.730 |
| Restrictive | 2.4 | 0.0 | 0.6 | 0.072 | 8.7 | 1.5 | 3.4 | 0.104 | 0.074 |
| Hypertrophic | 0.0 | 1.5 | 1.1 | 0.428 | 0.0 | 3.1 | 2.3 | 0.395 | 0.500 |
| Ischemic | 47.6 | 34.6 | 37.6 | 0.127 | 52.2 | 15.4 | 25.0 | 0.001 | 0.400 |
| Valvulopathy | 0.0 | 4.4 | 3.4 | 0.165 | 8.7 | 15.4 | 13.6 | 0.422 | <0.001 |
AF: atrial fibrillation; SR: sinus rhythm.
Concerning group B, no differences were found between patients with or without digoxin regarding demographic parameters, cardiovascular risk factors or comorbidities. LVEF was significantly lower in patients on digoxin therapy (25.0% vs. 30.0%, p=0.003) and admission creatinine was also lower (1.4±0.6 vs. 1.7±1.1mg/dl; p=0.028) (Table 2). Dilated cardiomyopathy was more frequent in patients on digoxin therapy (57.4% vs. 35.7%, p=0.014) (Table 3).
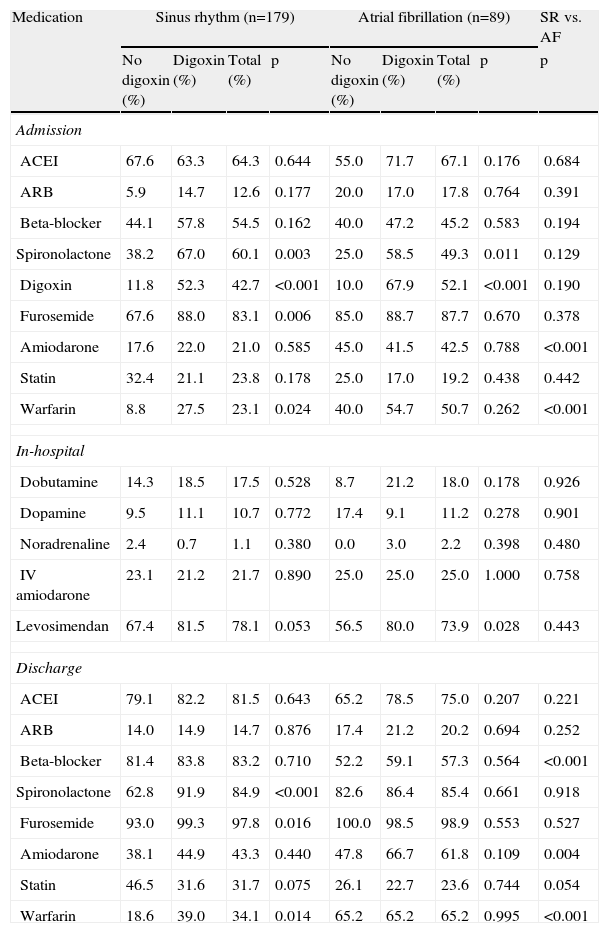
TherapyMedications on admission, during hospital stay and at discharge are described in Table 4. AF patients were more often medicated with amiodarone (42.5% vs. 21.0%, p=0.001) and warfarin (50.7% vs. 23.1%, p=0.001) on admission and at discharge (61.8% vs. 43.3%; p=0.004 and 65.2% vs. 34.1%; p=0.001, respectively, for amiodarone and warfarin) and the prescription of beta-blockers was significantly lower (57.3% vs. 83.2%; p=0.001) compared with patients in SR.
Medication on admission, during hospital stay and at discharge.
| Medication | Sinus rhythm (n=179) | Atrial fibrillation (n=89) | SR vs. AF | ||||||
| No digoxin (%) | Digoxin (%) | Total (%) | p | No digoxin (%) | Digoxin (%) | Total (%) | p | p | |
| Admission | |||||||||
| ACEI | 67.6 | 63.3 | 64.3 | 0.644 | 55.0 | 71.7 | 67.1 | 0.176 | 0.684 |
| ARB | 5.9 | 14.7 | 12.6 | 0.177 | 20.0 | 17.0 | 17.8 | 0.764 | 0.391 |
| Beta-blocker | 44.1 | 57.8 | 54.5 | 0.162 | 40.0 | 47.2 | 45.2 | 0.583 | 0.194 |
| Spironolactone | 38.2 | 67.0 | 60.1 | 0.003 | 25.0 | 58.5 | 49.3 | 0.011 | 0.129 |
| Digoxin | 11.8 | 52.3 | 42.7 | <0.001 | 10.0 | 67.9 | 52.1 | <0.001 | 0.190 |
| Furosemide | 67.6 | 88.0 | 83.1 | 0.006 | 85.0 | 88.7 | 87.7 | 0.670 | 0.378 |
| Amiodarone | 17.6 | 22.0 | 21.0 | 0.585 | 45.0 | 41.5 | 42.5 | 0.788 | <0.001 |
| Statin | 32.4 | 21.1 | 23.8 | 0.178 | 25.0 | 17.0 | 19.2 | 0.438 | 0.442 |
| Warfarin | 8.8 | 27.5 | 23.1 | 0.024 | 40.0 | 54.7 | 50.7 | 0.262 | <0.001 |
| In-hospital | |||||||||
| Dobutamine | 14.3 | 18.5 | 17.5 | 0.528 | 8.7 | 21.2 | 18.0 | 0.178 | 0.926 |
| Dopamine | 9.5 | 11.1 | 10.7 | 0.772 | 17.4 | 9.1 | 11.2 | 0.278 | 0.901 |
| Noradrenaline | 2.4 | 0.7 | 1.1 | 0.380 | 0.0 | 3.0 | 2.2 | 0.398 | 0.480 |
| IV amiodarone | 23.1 | 21.2 | 21.7 | 0.890 | 25.0 | 25.0 | 25.0 | 1.000 | 0.758 |
| Levosimendan | 67.4 | 81.5 | 78.1 | 0.053 | 56.5 | 80.0 | 73.9 | 0.028 | 0.443 |
| Discharge | |||||||||
| ACEI | 79.1 | 82.2 | 81.5 | 0.643 | 65.2 | 78.5 | 75.0 | 0.207 | 0.221 |
| ARB | 14.0 | 14.9 | 14.7 | 0.876 | 17.4 | 21.2 | 20.2 | 0.694 | 0.252 |
| Beta-blocker | 81.4 | 83.8 | 83.2 | 0.710 | 52.2 | 59.1 | 57.3 | 0.564 | <0.001 |
| Spironolactone | 62.8 | 91.9 | 84.9 | <0.001 | 82.6 | 86.4 | 85.4 | 0.661 | 0.918 |
| Furosemide | 93.0 | 99.3 | 97.8 | 0.016 | 100.0 | 98.5 | 98.9 | 0.553 | 0.527 |
| Amiodarone | 38.1 | 44.9 | 43.3 | 0.440 | 47.8 | 66.7 | 61.8 | 0.109 | 0.004 |
| Statin | 46.5 | 31.6 | 31.7 | 0.075 | 26.1 | 22.7 | 23.6 | 0.744 | 0.054 |
| Warfarin | 18.6 | 39.0 | 34.1 | 0.014 | 65.2 | 65.2 | 65.2 | 0.995 | <0.001 |
ACEI: angiotensin-converting enzyme inhibitor; AF: atrial fibrillation; ARB: angiotensin receptor blocker; IV: intravenous; SR: sinus rhythm.
In AF patients, spironolactone prescription on admission was higher in patients on digoxin therapy (58.5% vs. 25.0%; p=0.011), but no differences were found regarding other prior medications or discharge therapy.
Regarding SR patients, we observed that patients on digoxin therapy were more often medicated with spironolactone, furosemide and warfarin prior to hospital admission (67.0% vs. 38.2%; p=0.03; 88.0% vs. 67.6%; p=0.006; 27.5% vs. 8.8%; p=0.024, respectively) and at discharge (91.9% vs. 62.8%; p=0.001; 99.3% vs. 93.0%; p=0.016; 39.0% vs. 18.6%; p=0.014, respectively).
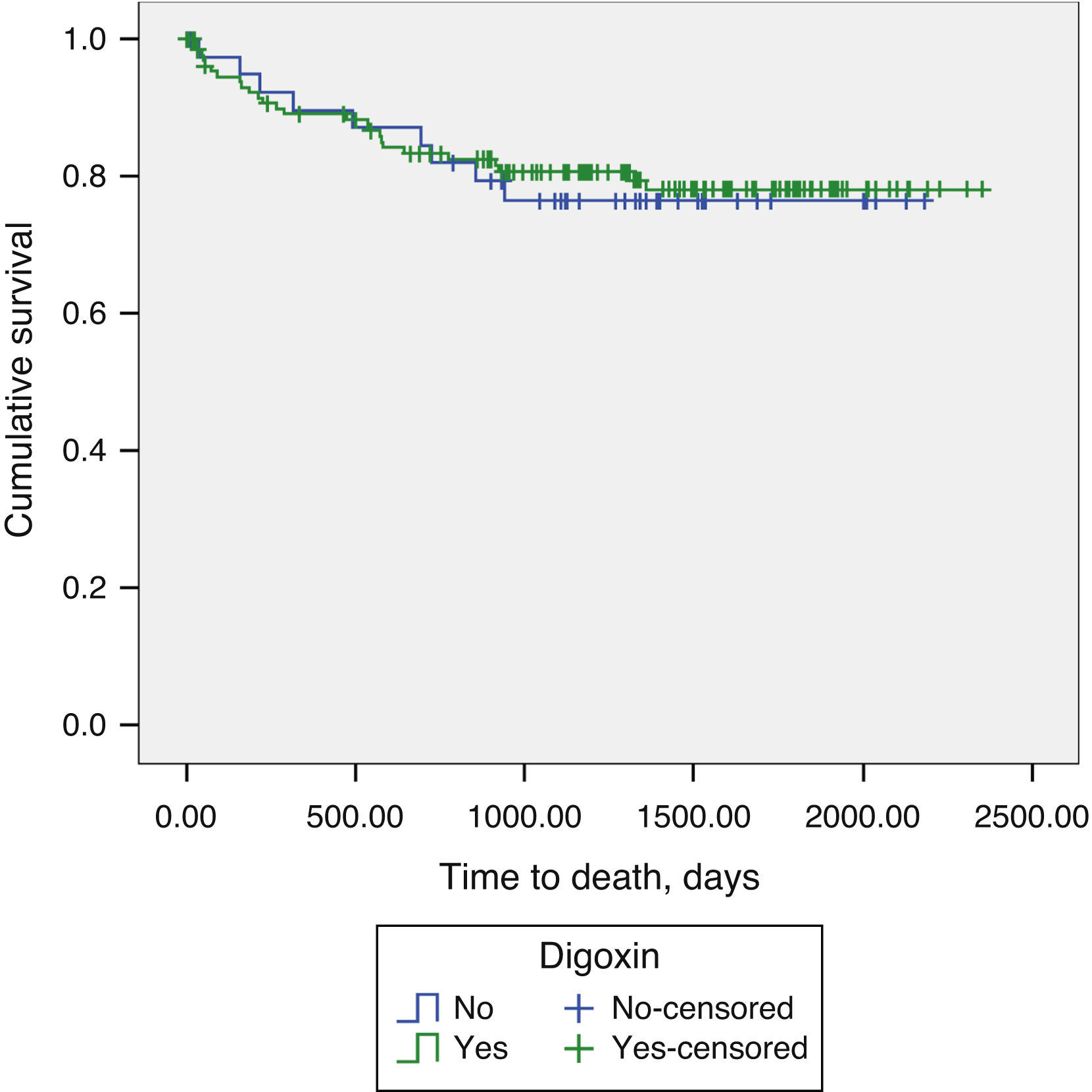
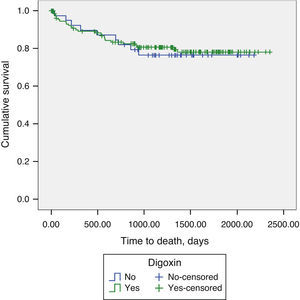
Long-term prognosisAbout one-fifth (21.1%) of patients underwent heart transplantation during the follow-up period. Addition of digoxin to contemporary standard HF therapy showed no impact on long-term mortality in patients with advanced HF and SR (19.1% vs. 22.5%, p=0.788) (Figure 1).
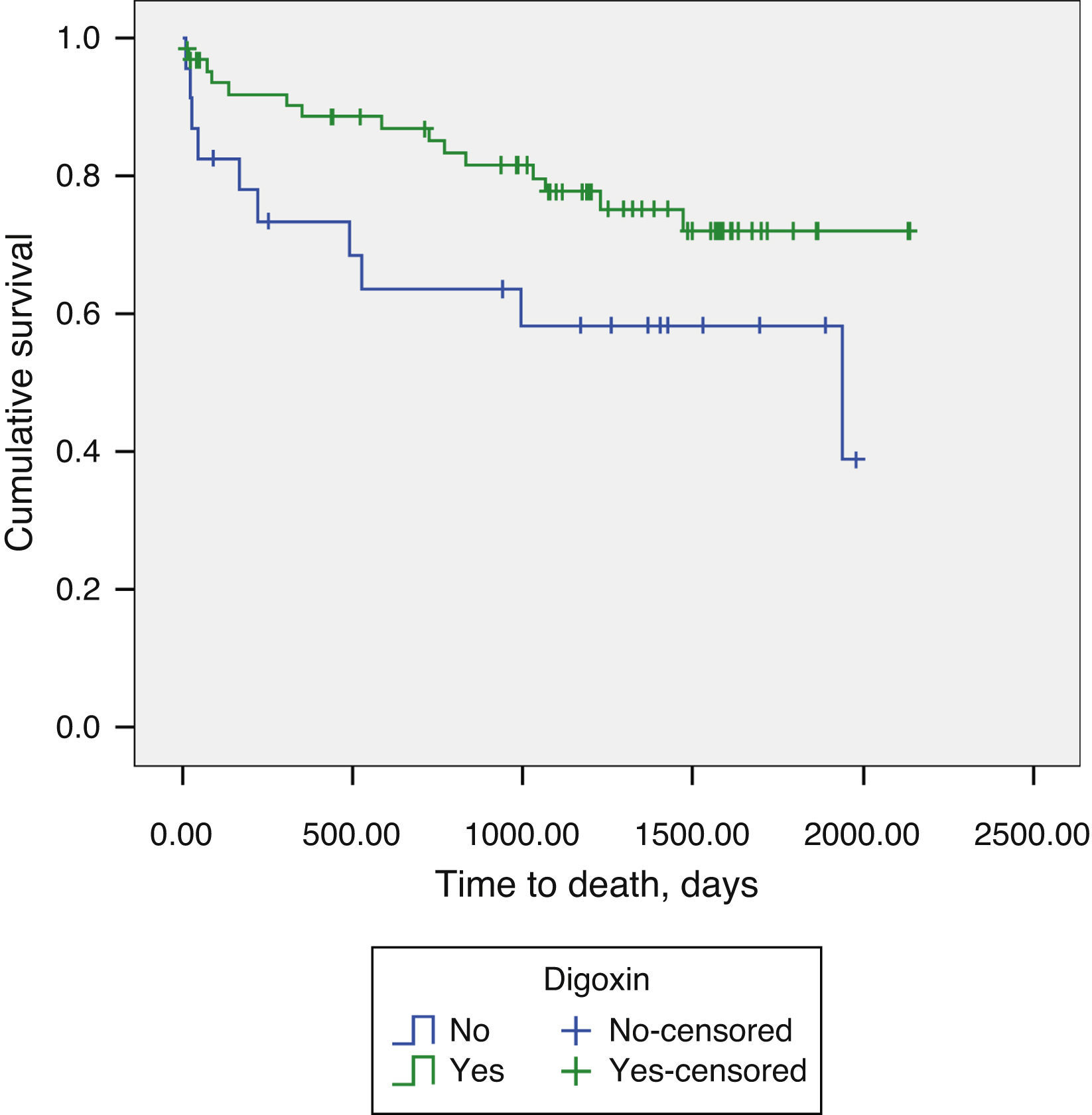
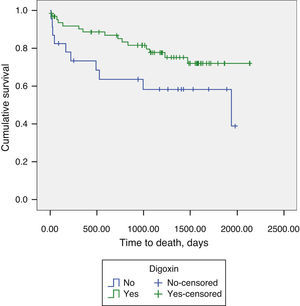
Regarding AF patients, we observed significantly lower long-term mortality in patients on digoxin therapy (18.6% vs. 46.6%, p=0.048) (Figure 2). Digoxin therapy did not influence the composite endpoint of all-cause mortality and readmissions for decompensated HF, in the total population or stratified by AF/SR.
DiscussionDespite recent advances in therapy, HF remains associated with high mortality and hospitalization rates, as we report in our contemporary cohort. Digoxin is the oldest HF drug and one of the least expensive. The ACC/AHA HF guidelines recommend considering adding digoxin in patients with persisting HF symptoms already treated with diuretics, an ACEI (or angiotensin receptor blocker) and a beta-blocker.1 However, there is limited evidence on the role of digoxin in patients on background therapy with beta-blockers and ACEIs.
The landmark DIG study, a randomized controlled trial of digoxin in patients with chronic HF already medicated with diuretics, showed that digoxin had no impact on overall mortality, but did reduce the rate of hospitalizations due to HF.9 However, the prescription rate of beta-blockers was not reported and, at the time the trial was conducted, beta-blockers were not accepted as standard therapy for patients with HF. Participants in the DIG trial were male outpatients with chronic stable HF (the majority in NYHA class I or II) in SR, and thus did not reflect real-world HF populations.9 Similarly to the DIG trial, we did not see a survival benefit in advanced HF patients in sinus rhythm. However, we failed to demonstrate a reduction in HF hospitalizations, probably due to lack of power.
Our population is significantly different from that of the DIG trial, with more severe patients hospitalized for decompensated HF, all in NYHA class III or IV with severely depressed LVEF, and a high prevalence of comorbidities. The severity of this population is highlighted by the significant proportion of patients who ultimately proceeded to heart transplantation. Furthermore, the use of diuretics, ACEIs, beta-blockers and devices in our study population was higher than in other studies examining the role of digoxin. This may have reduced the independent beneficial effect of digoxin on HF readmissions. The same is reported in a substudy of the Valsartan in Heart Failure Trial (Val-HeFT).12 In a cohort of 6800 patients, the investigators also failed to show any hospitalization benefit with the use of digoxin therapy, further supporting our results. One relatively recent retrospective study suggests that there was no benefit with digoxin in a heart transplant referral population of 455 patients with advanced HF receiving contemporary medical therapy.13 In this study, digoxin use was associated with increased risk of the primary outcome of death, urgent transplantation or ventricular assist device implantation, and the risk was higher in patients in SR compared with AF.
Our study suggests that digoxin therapy may improve the vital prognosis of patients with advanced HF and AF under optimal medical therapy. In AF patients, one potential mechanism of benefit of digoxin is simply to reduce ventricular rate, thereby limiting myocardial oxygen consumption, a concept in which interest was recently reawakened with the publication of the landmark SHIFT trial.14 The idea that beta-blockers might eliminate the need for digoxin in patients with AF and HF was the rationale for the CAFE study.11 However, rather than showing that digoxin is redundant, the study suggested that there could be synergistic benefits between digoxin and carvedilol.
There are reasons other than rate control why digoxin may be more effective in the presence of beta blockade. Beta-blockers, especially nonselective ones, that attenuate stress-induced reductions in serum potassium15 might neutralize the increase in sudden death associated with the use of digoxin. The inotropic effect of digoxin may improve tolerability to beta-blockers, enabling higher dose titration. Aldosterone antagonists also increase serum potassium, reducing the incidence of hypokalemia; it is well known that sudden death reduction was one of the primary mechanisms of mortality reduction in the Randomized Aldactone Evaluation Study (RALES)16 and the EPHESUS trial.17 Spironolactone tended to exert a greater reduction in mortality among patients treated with digoxin.
Post-hoc analyses of the DIG trial suggest that digoxin reduced mortality at low (0.5–0.9ng/ml) serum digoxin concentrations (SDC), but had no effect at higher (≥1ng/ml) SDC.18 A closer examination of the Kaplan–Meier survival plots in the DIG trial reveals an early mortality reduction in the digoxin group, followed by a later virtual overlap of the plots, suggesting that, although the early survival benefit was eliminated in later years, there was no increase in mortality.19 This lack of a long-term effect of digoxin on mortality may be due to use of open-labeled digoxin in the placebo group, and a cumulative effect of the use of high-dose digoxin; more than 80% of the participants in the DIG trial were receiving ≥0.25mg of digoxin daily or matching placebo, which was higher than the currently recommended daily dosage.18 In our population, the standard daily dose was 0.125mg, with a majority of patients pausing on weekends; the maximum dose prescribed during data collection was 0.25mg/day, five times a week. The continued use of high-dose digoxin in elderly patients, with deteriorating kidney function, may have resulted in higher SDC during the later years of the DIG trial. The beneficial effects of digoxin at low SDC are primarily due to its effect on the neurohormonal system.18 By inhibiting the sodium-potassium adenosine triphosphate pump in renal tubules and vagal afferent fibers, digoxin suppresses both the renin-angiotensin-aldosterone and sympathetic nervous systems.18 Low SDC also reduces the risk of digoxin toxicity and associated morbidity and mortality.18 Concomitant therapy with beta-blockers and aldosterone antagonists and use of lower doses of digoxin could radically improve the risk/benefit ratio for digoxin.20
The explanation for different outcomes in AF and SR remains elusive. In our population, we observed significantly lower LVEF in patients in SR (26.0% vs. 31.0%, p=0.003), but no other significant differences were found between patients in SR and AF.
Our study has several limitations. First, this observational retrospective study reflects a single-center experience and includes a relatively small number of patients. Second, we did not have data for SDC; however, the standard dose prescribed was 0.125mg/day five times a week and the maximum dose prescribed during data collection was 0.25mg/day, and we know that digoxin dose is a strong predictor of SDC. Given the nature of the data set, we were unable to ascertain the length of therapy on digoxin before the index HF admission. Third, data regarding discontinuing or starting therapy, or optimization of drug doses during follow-up, were not available. Previous studies on digoxin in patients with chronic HF reported improvements in LVEF, HF symptoms and exercise performance. In our study, these were not measured, and thus we cannot exclude potential beneficial effects on these soft endpoints. One issue with retrospective outcomes analysis is always whether or not the more severe patients preferentially received a given therapy. However, in our population there were no significant differences between AF patients with or without digoxin regarding demographic parameters, significant comorbidities, or hemodynamic, laboratory, electrocardiographic and echocardiographic data on admission, making selection bias unlikely.
ConclusionsThe present study suggests that digoxin may improve the vital prognosis of AF patients with advanced HF under optimal medical therapy. Digoxin did not demonstrate benefit in patients in SR. These results may help to improve patient selection for treatment with digoxin, in order to achieve a better risk/benefit ratio in advanced HF patients. Polypharmacy is known to be associated with an increased risk of adverse drug reactions, drug interactions and poor compliance. Thus, for multiple reasons of both efficacy and safety, whether or not digoxin therapy has any role in the current management of advanced HF needs to be evaluated. Further randomized controlled trials are required to find out whether digoxin has a role in contemporary management of HF.
Conflicts of interestThe authors have no conflicts of interest to declare.