Sudden cardiac death (SCD) can be the first clinical manifestation of Wolff–Parkinson–White (WPW) syndrome.
Catheter ablation of accessory pathways is now a safe and effective procedure, and is widely recommended in patients with WPW syndrome. However, management of the asymptomatic WPW patient remains controversial. Recent studies have readdressed the issue of risk stratification and prophylactic catheter ablation.
We describe a case of malignant arrhythmia and aborted SCD as first presentation of WPW syndrome in a previously asymptomatic 17-year-old patient.
A morte súbita cardíaca (MSC) pode ser a primeira manifestação da síndrome de Wolff–Parkinson–White (WPW).
A ablação por cateter da via acessória é atualmente um tratamento seguro e eficaz, estando liberalmente recomendado em doentes sintomáticos. Já na presença de padrão electrocardiográfico de WPW, a orientação terapêutica é alvo de controvérsia. Alguns estudos vieram reativar a discussão relativamente à estratificação de risco e benefício da ablação profilática.
Descrevemos o caso clínico de um jovem de 17 anos previamente assintomático, com arritmia maligna e morte súbita cardíaca abortada como primeira manifestação da doença.
atrial fibrillation
accessory pathway
accessory pathway antegrade effective refractory period
atrioventricular
atrioventricular reciprocating tachycardia
coronary care unit
electrocardiogram
emergency department
electrophysiological study
radiofrequency ablation
sudden cardiac death
ventricular fibrillation
Wolff–Parkinson–White
WPW syndrome is a disorder characterized by the presence of one or more accessory pathways that predispose patients to frequent episodes of arrhythmias.1 The 2003 ESC/ACC/AHA guidelines2 recommend routine electrophysiological study (EPS) with liberal indications for catheter ablation in symptomatic patients. However, management of asymptomatic subjects with incidentally found pre-excitation patterns remains controversial. Prognosis is usually good, but there is a lifetime risk of malignant arrhythmias and sudden cardiac death (SCD), and the latter can be the first presentation of the disease. Although risk factors for fatal arrhythmic events are not well established, EPS can be a useful tool in risk stratification.3 A short accessory pathway anterograde effective refractory period (AP-AERP), inducibility of sustained tachyarrhythmias (atrioventricular reciprocating tachycardia [AVRT] and/or atrial fibrillation [AF]) and the presence of multiple accessory pathways are the strongest predictors of life-threatening arrhythmias and SCD.3–6
Case reportA 17-year-old male with no history of cardiovascular disease or familial SCD presented to the emergency department (ED) with palpitations. No medication or drug abuse was reported. Symptoms had started three hours earlier at rest. The physical examination revealed normal blood pressure (130/70mmHg) and an irregular pulse approaching 200bpm. The rest of the physical evaluation was unremarkable, with no cardiac murmurs or signs of pulmonary edema. An electrocardiogram (ECG) showed a wide-complex irregular tachycardia with rapid ventricular rate (Figure 1), suggesting pre-excited AF. Continuous heart monitoring was initiated and two venous lines were inserted.
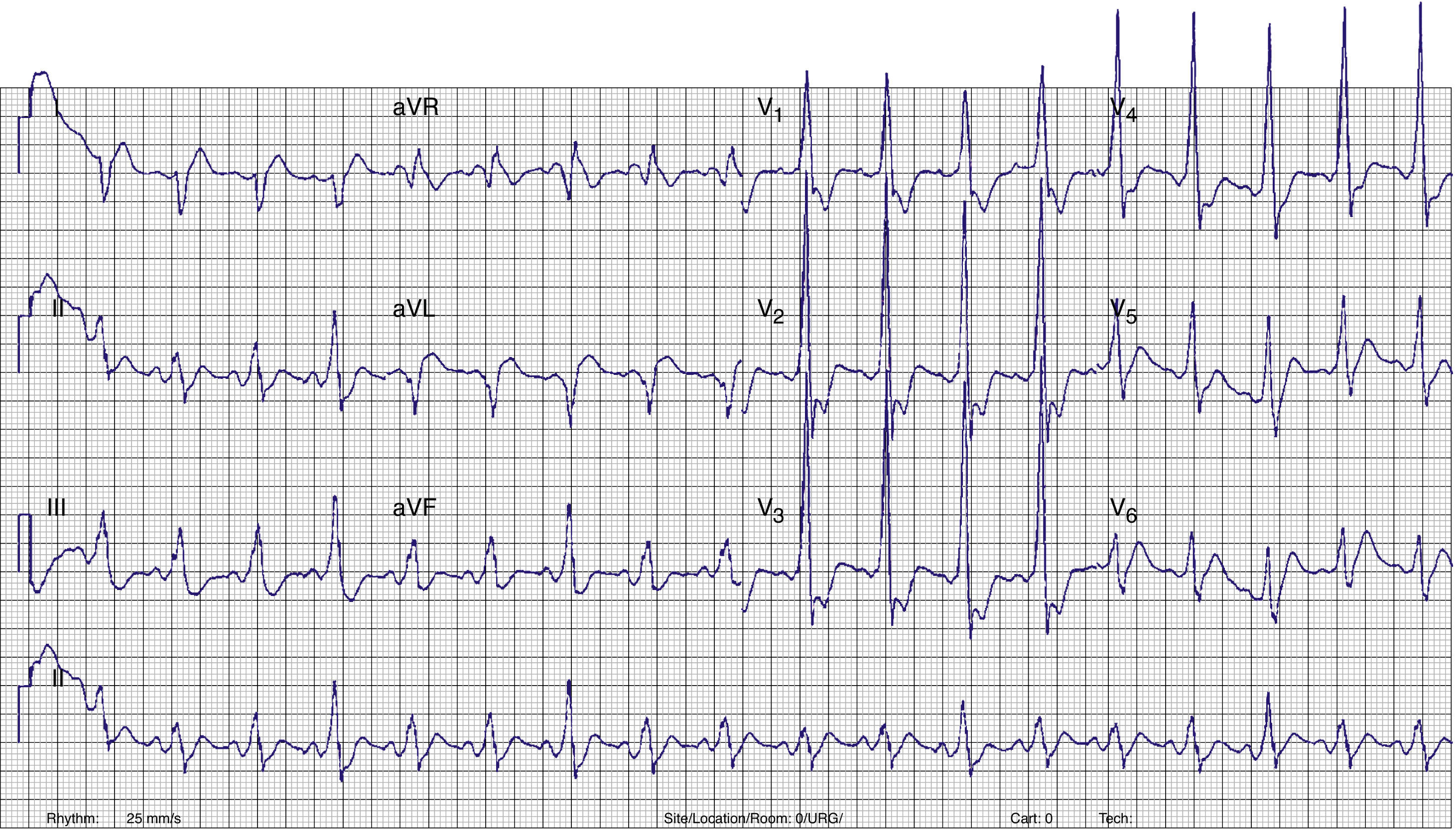
A few minutes after admission to the ED, the rhythm degenerated into ventricular fibrillation (VF) (checked on the monitor) and the patient collapsed without pulse. Cardiopulmonary resuscitation was promptly started. Recovery of regular pulse and rhythm was confirmed after defibrillation with two electrical shocks (2× 150J, biphasic). The ECG then obtained revealed sinus rhythm with ventricular pre-excitation (shortened PR interval, widened QRS complex with delta wave and secondary ventricular repolarization abnormalities) (Figure 2). The patient was admitted to the coronary care unit (CCU). No rhythm abnormalities were recorded during CCU monitoring. Serum potassium and magnesium levels were normal and transthoracic echocardiography excluded structural heart disease.
Twelve-lead ECG after conversion to sinus rhythm. Shortened PR interval, delta wave, widened QRS complex and secondary repolarization abnormalities are seen. This ECG is indicative of WPW syndrome, and a left lateral AP is suggested (positive delta wave in V1 and inferior leads and negative delta wave in aVL and DI).13
EPS was scheduled and performed within 12hours of admission. Two catheters were positioned via the right femoral vein: a deflectable decapolar catheter in the coronary sinus, and a nondeflectable quadripolar catheter in the right ventricle for His activity tracing and ventricular stimulation. Atrial stimulation was performed using the decapolar catheter. A short AP-AERP was recorded (210ms) using programmed atrial stimulation, indicating a high-risk AP.
A 4-mm ablation catheter (RF Mariner™, Medtronic Inc., Minneapolis, USA) was advanced retrogradely via the right femoral artery and placed in the mitral ring.
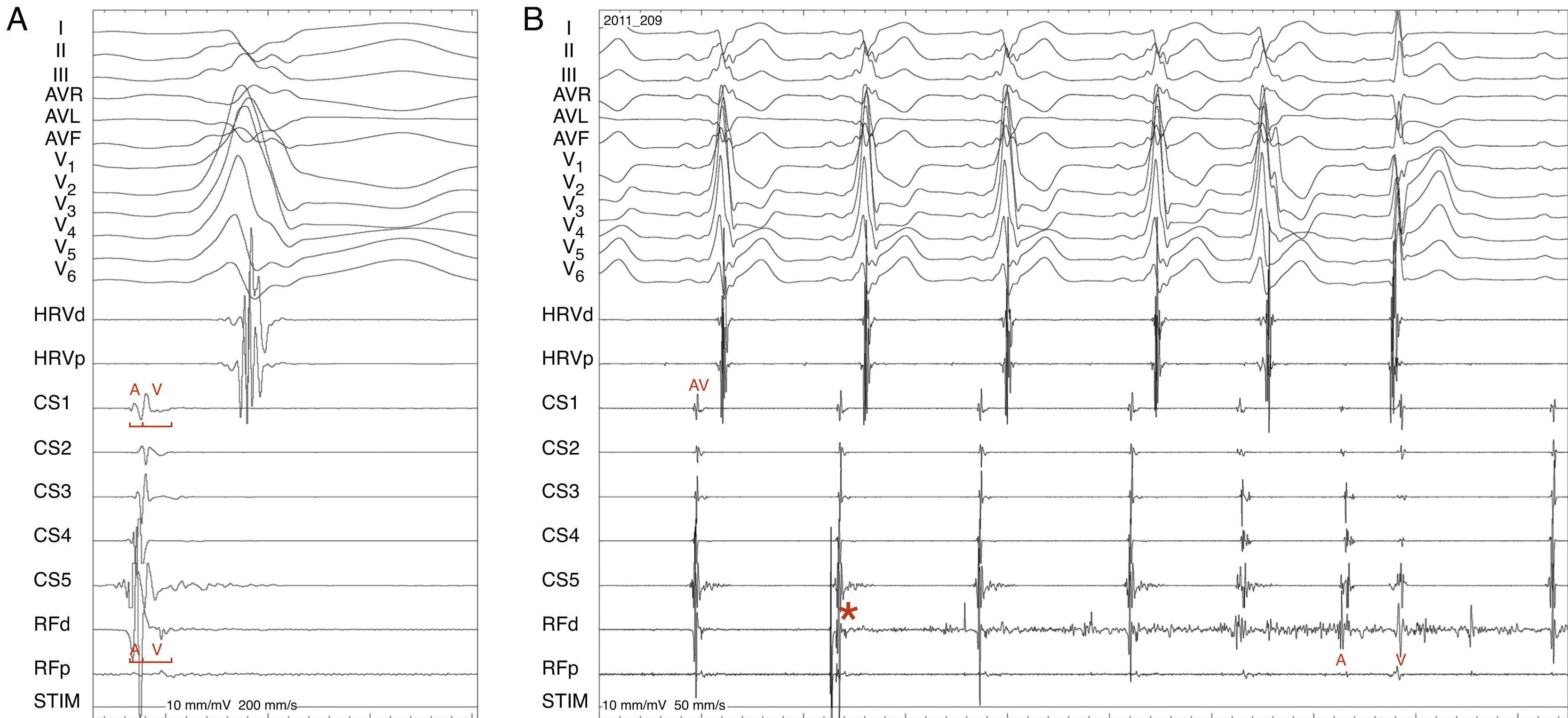
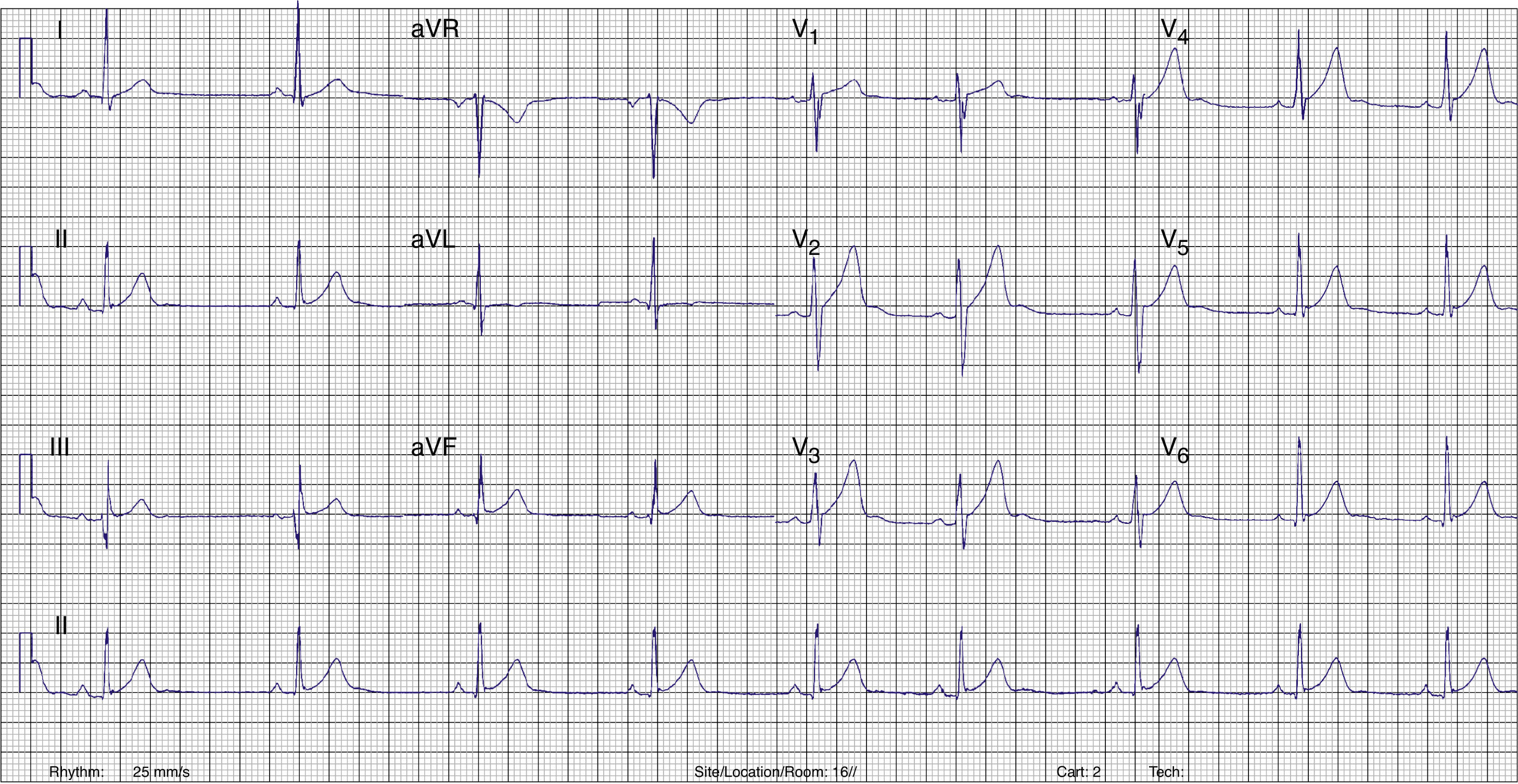
Radiofrequency energy (50W/70°C) was applied for 60s to the atrial side of the left lateral mitral ring. Conduction over the AP was successfully interrupted within 3s of energy delivery (Figure 3). During the procedure, AF without pre-excitation was triggered, and sinus rhythm was spontaneously recovered. Total procedure time was 90min, and total fluoroscopy time was 17min. The ECG tracing after catheter ablation showed PR and QRS intervals within normal limits (Figure 4). No procedural complications ensued and the patient was discharged three days after admission. Six months after catheter ablation the patient was asymptomatic with a normal ECG tracing. No tachyarrhythmias were documented during this period.
Accessory pathway ablation. Surface leads I, II, III, aVF, aVL, aVF and V1–V6 are shown, with intracardiac recordings from catheters in the right ventricle (HRVp and HRVd), distal (CS1) and proximal (CS5) coronary sinus, and proximal (RFp) and distal (RFd) ablation catheter. Atrial (A) and ventricular (V) electrical signals are shown. (A) Electrocardiographic and intracardiac signals immediately before catheter ablation, recorded at 200mm/s. (B) Catheter accessory pathway ablation, recorded at 50mm/s. Conduction over the AP disappeared within 3s of radiofrequency energy application. The asterisk represents the beginning of radiofrequency energy application.
WPW is a cardiac conduction disorder characterized by the presence of one or multiple APs that predispose patients to frequent episodes of arrhythmia. A Wolff–Parkinson–White pattern is present in 0.1–0.2% of the general population,7 most of whom will never be aware of the issue unless it is discovered incidentally. Symptomatic patients generally experience a good outcome, with either no recurrent arrhythmias or only benign recurrences. Risk of SCD is low, with annual estimates of 0.1% for asymptomatic8 and 0.15–0.39% for symptomatic patients.2
The ECG features of WPW include a PR interval of <0.12s, slurring and slow rise of the initial QRS complex (delta wave), a widened QRS complex with a total duration greater than 0.12s, and secondary repolarization abnormalities that are generally directed in an opposite direction to the major delta and QRS vectors. Diagnosis of WPW syndrome requires typical ECG findings with a documented dysrhythmia. The most frequently encountered dysrhythmia in patients with WPW is atrioventricular reciprocating tachycardia (AVRT), which occurs in 80% of cases.1 AF is not uncommon, occurring in 15–30% of patients.2 This is a potentially life-threatening arrhythmia in patients with WPW syndrome and may lead to SCD. If an AP has a short anterograde refractory period, rapid repetitive conduction to the ventricles during AF can result in rapid ventricular response with subsequent degeneration to VF.
Predicting clinical outcome is one of the major issues in asymptomatic WPW subjects. Risk assessment is not well defined and remains a considerable clinical challenge.
Risk factors for potentially life-threatening arrhythmic events in WPW3–6 include: (1) short AP-AERP (<250ms) allowing a rapid ventricular response in AF; (2) inducibility of tachyarrhythmia during EPS (AVRT and/or AF); (3) short pre-excited RR interval during AF (<250ms); (4) multiple APs; (5) male gender; (6) age; and (7) syncope.
Invasive EPS most accurately assesses the electrophysiological properties of the AP and its role in the patient's clinical arrhythmia, although no single factor has high sensitivity, specificity and positive predictive value.9 Pappone et al.3–5,10 reported that a particular subgroup of asymptomatic patients may be at risk for a malignant arrhythmic event and demonstrated the value of EPS in stratifying asymptomatic patients into high- and low-risk groups.
Noninvasive markers of lower risk such as intermittent loss of pre-excitation, loss of AP conduction on exercise stress testing, and sudden loss of AP conduction after treatment with the antiarrhythmic drugs procainamide or flecainide are limited by insufficient sensitivity and specificity, and currently play little role in patient management.11
In our case of a previously healthy 17-year-old patient there were no past ECG records and thus the existence of an AP was not known. A malignant arrhythmia with degeneration to VF was the first presentation and could have resulted in SCD. The first-line therapeutic option was EPS before hospital discharge, resulting in successful ablation of the high-risk AP. However, had the WPW pattern been incidentally found before symptoms, how should we have proceeded?
The 2003 ESC/ACC/AHA guidelines2 are restrictive regarding the management of an asymptomatic WPW pattern, and recommend routine invasive EPS and catheter ablation only in symptomatic patients. This may be questionable: catheter ablation is now routinely and safely performed by skilled operators, and asymptomatic patients are more commonly referred for invasive risk stratification and prophylactic AP ablation. Pappone et al.5,10 reported that prophylactic AP catheter ablation performed at the time of initial EPS improved the long-term outcome of patients at high risk for malignant arrhythmias, and the risk significantly and persistently decreased over time after ablation. Furthermore, the efficacy of catheter AP ablation approaches 100%, and overall procedure-related mortality is less than 0.2%.2,12 Accordingly, radiofrequency catheter ablation (RFA) is now widely accepted as a therapy for WPW and is frequently considered the first-line therapy. However, different AP locations still represent challenges for ablation. Parahisian and midseptal APs, which account for only a minority of cases, pose a significant challenge to RFA due to their proximity to the His bundle and AV node, increasing the risk of AV block. A possibly safer approach for elimination of these challenging APs is cryoablation, which creates homogeneous and smaller lesions and is less thrombogenic than RFA, reducing the risk of inadvertent AV block.14 The potential risk of AV block due to RFA of parahisian and midseptal APs must always be discussed with the patient, particularly in the younger, and balanced against the benefits of ablation. In conclusion, the benefits of prophylactic catheter ablation (RFA or cryoablation) can outweigh the procedural risks when performed by a skilled operator, and the issue of the management of asymptomatic WPW patients could be readdressed.
Our case illustrates how a WPW pattern may not be as benign as thought. Sudden cardiac death is a dramatic occurrence, particularly in young healthy subjects, and is a real-life event and not merely an item on the reference list of a report on the natural history of the disease. If our patient had had a previous ECG tracing showing a WPW pattern, and if the guidelines had been followed, his fate would have been SCD in the absence of prompt medical assistance. This scenario must be always taken into consideration in each patient with an incidental WPW pattern.
WPW-associated deaths are preventable given the availability of a permanent treatment that is safe and effective, and the benefits of catheter ablation are likely to outweigh the procedural risks when performed by a skilled operator.
The latest guidelines2 were published over nine years ago. The authors consider that it is currently unacceptable that even one asymptomatic patient with WPW pattern should die or experience a life-threatening arrhythmic event due to a high-risk accessory pathway.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.


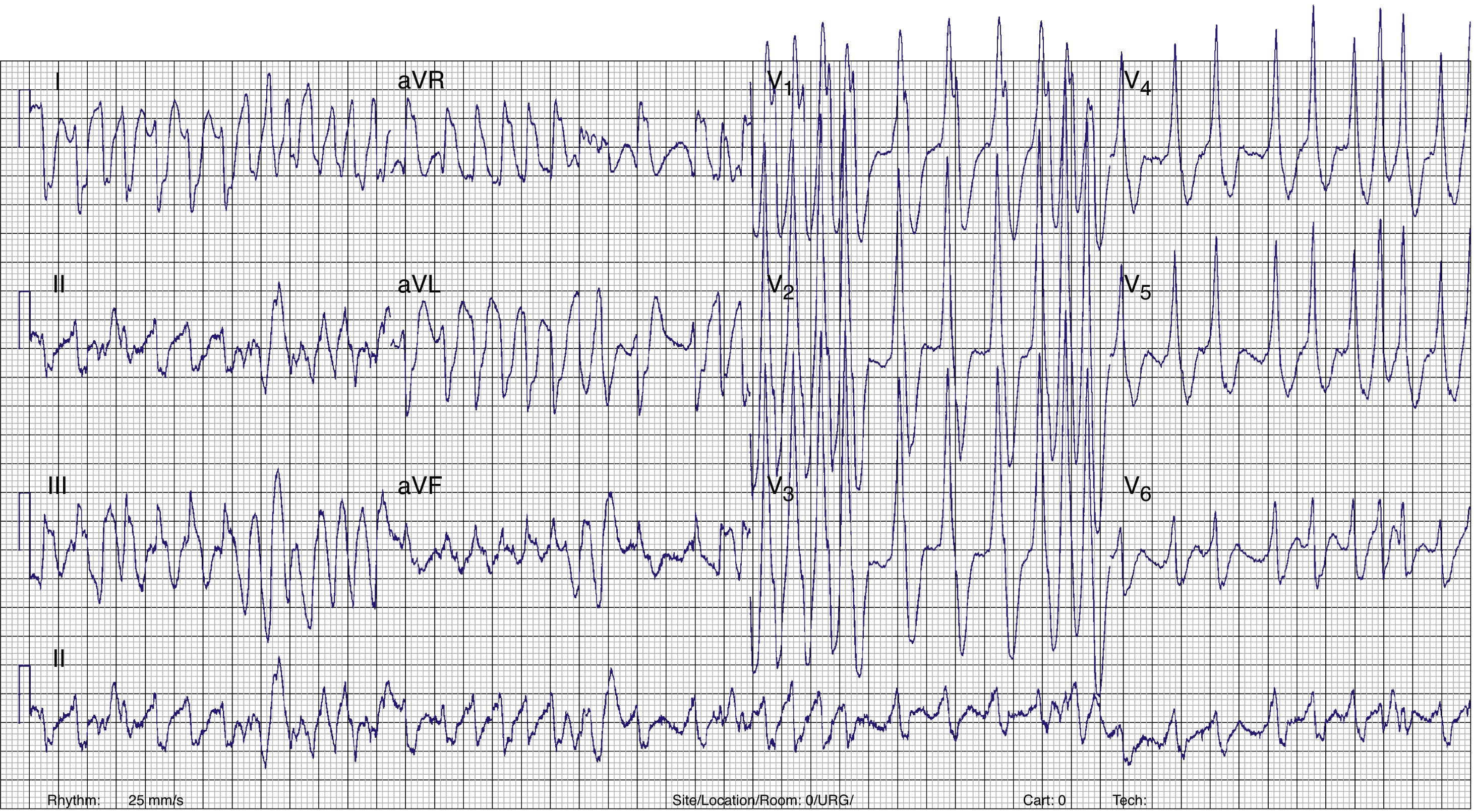
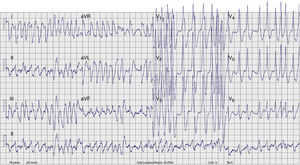 ECG. A wide-complex irregular tachycardia is shown consistent with pre-excited AF. The ventricular response is very rapid, and the shortest pre-excited RR interval is nearly 200ms.' title='Presenting 12-lead
ECG. A wide-complex irregular tachycardia is shown consistent with pre-excited AF. The ventricular response is very rapid, and the shortest pre-excited RR interval is nearly 200ms.' title='Presenting 12-lead 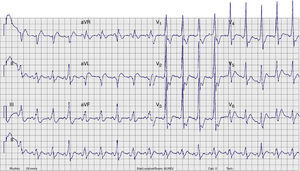 ECG after conversion to sinus rhythm. Shortened PR interval, delta wave, widened QRS complex and secondary repolarization abnormalities are seen. This
ECG after conversion to sinus rhythm. Shortened PR interval, delta wave, widened QRS complex and secondary repolarization abnormalities are seen. This 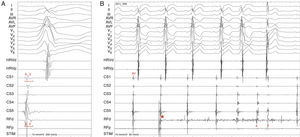
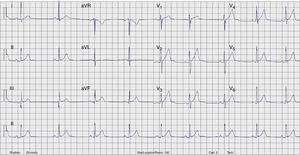 ECG after catheter ablation. PR and QRS intervals are within normal limits.' title='Twelve-lead
ECG after catheter ablation. PR and QRS intervals are within normal limits.' title='Twelve-lead 

