We report the case of a 34-year-old man with aortic valve infective endocarditis caused by methicillin-resistant Staphylococcus aureus, complicated by an aortic annular abscess. A 23-mm St. Jude HP aortic mechanical prosthesis was implanted. The pre-discharge transthoracic echocardiogram revealed a mycotic aneurysm of the basal posteroinferior wall, confirmed by cardiac magnetic resonance imaging, and it was decided to reintervene. The aneurysm was closed with a patch and the mitral valve had to be replaced.
Although a small leak from the aneurysm patch persisted on the pre-discharge transesophageal echocardiogram, there was no trace of the aneurysm at nine-month re-evaluation.
This case illustrates a rare complication of aortic valve endocarditis and shows the evolution of the mycotic aneurysm after closure via a transmitral approach.
Homem de 34 anos com endocardite infeciosa da válvula aórtica causada por estafilococo aureus meticilino resistente (MRSA), complicada por abcesso do anel aórtico. Na cirúrgica foi implantada prótese aórtica mecânica St. Jude 23HP. O ETT antes da alta evidenciou um aneurisma micótico no segmento basal da parede inferior do ventrículo esquerdo, confirmado por ressonância magnética cardíaca, tendo sido decidido reintervir. O aneurisma foi encerrado com retalho de pericárdio mas a válvula mitral teve de ser substituída.
Apesar de persistir uma pequena fuga no ETE antes da alta, verificou-se resolução total da lesão no exame de reavaliação realizado nove meses depois.
Este caso documenta uma complicação muito rara da endocardite da válvula aórtica e mostra a evolução do aneurisma micótico após encerramento por via transmitral.
Aortic valve endocarditis can be complicated by perivalvular extension – abscess, pseudoaneurysm and/or fistula. Systemic embolism and mycotic aneurysms have also been reported. Perivalvular extension most commonly occurs in the context of methicillin-resistant Staphylococcus aureus (MRSA) infection or prosthetic endocarditis, and is associated with increased morbidity and mortality.1,2
Mycotic aneurysms can be extracardiac (splenic, renal, cerebral or vascular) or intracardiac, the latter normally affecting the mitral or aortic valve apparatus and intervalvular fibrosa.1 Involvement of the left ventricular (LV) free wall is rare, with few cases described in the literature.3–6
Case reportA 34-year-old Caucasian man presented at his local hospital with peripheral petechiae and neurological symptoms. Brain CT revealed parenchymal cerebral hemorrhage requiring decompressive craniectomy. In view of suspected endocarditis, transesophageal echocardiography (TEE) was performed, which showed multiple vegetations on a bicuspid aortic valve, with a periannular abscess (6mm×13mm) located at 11 o’clock (surgical view). Abdominal CT revealed renal and splenic abscesses and blood cultures were positive for MRSA.
The patient was then referred to our surgical center with a diagnosis of aortic valve endocarditis with severe aortic regurgitation. A 23-mm St. Jude HP aortic mechanical prosthesis was implanted after the abscess was drained and closed with a pericardial patch. Intraoperative TEE confirmed exclusion of the abscess cavity and normal prosthetic function.
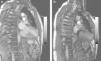
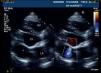
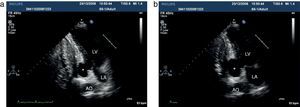
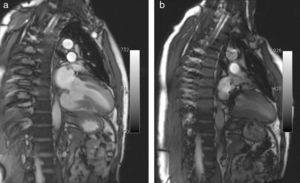
Pre-discharge transthoracic echocardiography (TTE) performed after six weeks of antibiotic therapy with meropenem and vancomycin showed a large mycotic aneurysm of the LV basal inferior wall adjacent to the posterior mitral annulus (Figure 1), which was confirmed by cardiac magnetic resonance imaging (CMRI) (Figure 2a). Even though the morphology of the aneurysm did not suggest an ischemic etiology, coronary angiography was performed, which confirmed normal epicardial coronary arteries.
Apical two-chamber view, showing a mushroom-shaped aneurysm in the left ventricular basal inferior wall (x) opening into the LV immediately below the posterior mitral annulus. Note the increase in size from end-diastole (a) to end-systole (b). AO: descending thoracic aorta; LA: left atrium; LV: left ventricle.
Although there was no clinical or laboratory evidence of continuing infection, it was decided to reintervene since the aneurysm's thin wall and high pulsatility indicated imminent rupture.
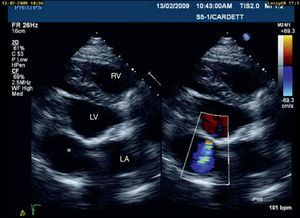
The aneurysm was closed with a bovine pericardial patch but its proximity to the posterior mitral annulus meant that the mitral valve had to be replaced with a 27-mm St. June mechanical prosthesis. Intraoperative TEE showed a small leak from the patch, which it was decided not to correct since the flow by color Doppler was minimal.
The postoperative period was uneventful and the patient was discharged ten days after surgery. On TTE one month after the intervention, a small aneurysmal sac (3cm×3cm) persisted with a small leak from the patch (Figure 3); it was decided to maintain close echocardiographic monitoring. At nine-month re-evaluation, TTE showed no trace of the aneurysm and contrast injection revealed no flow out of the ventricular chamber, which was confirmed by CMRI (Figure 2b).
DiscussionMyocardial infarction is by far the most common cause of LV aneurysm.1 Mycotic aneurysms are rare,2–5 and other uncommon causes include cardiac trauma, Chagas disease, sarcoidosis and congenital etiology.1
Three mechanisms have been described for the formation of mycotic aneurysms of the LV wall: septic coronary embolism leading to infarction and rupture into the ventricular chamber; dissemination from an adjacent perivalvular abscess; and seeding of the endocardium by a regurgitant jet.4
In the case presented, the recent history of MRSA endocarditis, the fact that the aneurysm was not present at the time of the first surgery, and the normal coronary arteries, all point to an infectious etiology. The absence of involvement of the mitral-aortic fibrosa or the mitral valve apparatus rules out infection from adjacent structures. While the possibility of septic coronary embolism causing myocardial infarction must be considered, the evolution of the aneurysm in the postoperative period and its morphology make this unlikely. We therefore believe that the causal mechanism in this patient was the seeding of the endocardium, leading to myocardial infection and wall ulceration.
LV mycotic aneurysms can rupture into the pericardium and cause tamponade,4,5 or they can heal without requiring intervention.6 In our patient, the size of the aneurysm, the thinness of its wall and presumed rapid evolution were taken as signs of imminent rupture, which prompted the decision for surgical treatment. Replacement of the mitral valve was the price paid to exclude the aneurysm. Nevertheless, resolution of the LV infection, with minimal residual scarring and impairment of LV function, leads us to believe that surgical treatment was the best option in this case.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Correia, E. Aneurisma micótico da parede livre do ventrículo esquerdo como complicação de endocardite da válvula aórtica. doi:10.1016/j.repc.2011.10.010.