Marfan syndrome is an autosomal dominant connective tissue disease with an estimated incidence of 1 in 5000 individuals. In 90% of cases it is caused by mutations in the gene for fibrillin-1, the main constituent of extracellular microfibrils. Studies on animal models of Marfan syndrome have revealed that fibrillin-1 mutations interfere with local TGF-β signaling, in addition to impairing tissue integrity. The cardinal features involve the cardiovascular, ocular and skeletal systems. The diagnosis of Marfan syndrome is made according to the revised Ghent nosology. Early identification and appropriate management are critical for patients with Marfan syndrome, who are prone to the life-threatening cardiovascular complications of aortic aneurysms and aortic dissection. The standard treatment includes prophylactic beta-blockers in order to slow down dilation of the ascending aorta, and prophylactic aortic surgery. The success of current medical and surgical treatment of aortic disease in Marfan syndrome has substantially improved mean life expectancy, extending it above 72 years. This review aims to provide an overview of this hereditary disorder.
A síndrome de Marfan é uma doença autossómica dominante do tecido conjuntivo, com uma incidência estimada de um em 5000 indivíduos. Em 90% dos casos resulta de mutações do gene da fibrilina-1, principal componente das microfibrilas da matriz extracelular. Estudos em modelos animais da síndrome de Marfan demonstraram que as mutações da fibrilina-1 interferem com a via de sinalização do TGF-β, além de comprometer a integridade do tecido conjuntivo. As principais manifestações envolvem os sistemas cardiovascular, ocular e esquelético. O diagnóstico da síndrome de Marfan é feito de acordo com a nova revisão da nosologia de Ghent. Um diagnóstico precoce e tratamento adequado são fundamentais para os doentes com síndrome de Marfan que estão sujeitos às complicações cardiovasculares potencialmente fatais dos aneurismas da aorta e da disseção aórtica. O tratamento padrão inclui β-bloqueantes profiláticos para diminuir a taxa de dilatação da aorta ascendente e cirurgia profilática da aorta. O sucesso do tratamento médico e cirúrgico atual da patologia aórtica da síndrome de Marfan melhorou substancialmente a esperança média de vida, estendendo-a acima dos 72 anos. Esta revisão visa proporcionar uma visão global desta doença hereditária.
Marfan syndrome (MFS) is an autosomal dominant connective tissue disease with an estimated incidence of 1 in 5000 individuals.1,2 It exhibits complete penetrance, variable interfamilial and intrafamilial expressivity, and pleiotropy, and shows no predilection for gender, race, ethnicity, or geographical location.1,3 Around a quarter of cases are caused by a new mutation, while the remaining three-quarters inherit the condition from an affected parent.3,4
The Berlin criteria for clinical diagnosis of MFS were established in 1986, and were replaced in 1996 by the Ghent criteria, which were updated to the most recent version in 2010.5,6
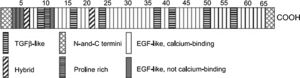
GeneticsIn 90% of cases7,8 classical MFS is caused by mutations in FBN1, located on chromosome 15q21.1 and containing 65 exons. This gene codes for the glycoprotein fibrillin-1, which is the main constituent of extracellular microfibrils9 and is found in the skin, lungs, kidneys, blood vessels, cartilage, tendons, muscles, cornea, and ciliary zonules.10 It is composed of 47 repeating domains homologous to epidermal growth factor, 43 of which are homologous to calcium-binding epidermal growth factor (cbEGF).9 Each domain contains six cysteine residues that form disulfide bridges between C1 and C3, C2 and C4, and C5 and C6, which stabilize its three-dimensional conformation.10 The cbEGF modules play an important part in protecting fibrillin against proteolysis, promoting interactions between fibrillin monomers and other components of microfibrils, and stabilizing the structure of microfibrils.11,12 Fibrillin-1 also contains seven domains homologous to latent transforming growth factor binding protein (LTBP), as well as a hybrid module with characteristics of both cbEGF and LTBP (Figure 1).9,11–13
Schematic representation of the organization of the gene for fibrillin-1 (adapted from Nollen et al.52). EGF-like: domains homologous to epidermal growth factor; TGF-β-like: domains homologous to those in latent transforming growth factor-beta.
Point mutations are the type most commonly found in MFS, of which 60% are missense and 10% are nonsense. The most frequent missense mutation results from substitution of cysteine residues, which interferes with the normal function of the sulfide bridges in cbEGF and LTBP.9,13
Mutations in the FBN1 gene do not appear to present genotype/phenotype correlation.10,13–15 Their high degree of interfamilial and intrafamilial variability suggests that environmental factors, stochastic effects and modifying genes may also influence phenotype expression. The level of expression of the normal FBN1 allele and hyperhomocysteinemia associated with the C677T polymorphism of methylenetetrahydrofolate reductase have been proposed as factors that modify the clinical severity of MFS.13
Various studies have set out to determine genotype-phenotype correlations, the strongest of which to date are related to neonatal MFS associated with mutations in exons 24-32 of FBN1, which leads to the severest form of the disease and manifests immediately after birth. These newborns show common characteristics of MFS such as arachodactyly and scoliosis, but also certain features specific to this phenotype, including crumpled ears, congenital flexion contractures, emphysema, and loose redundant skin. Survival beyond the age of 24 months is very rare; the main cause of death is congestive heart failure associated with mitral and tricuspid regurgitation.16,17
FBN1 mutations are also reported in individuals with phenotypes that are clinically similar to those of MFS but that do not meet the Ghent criteria. These include the following fibrillinopathies: ectopia lentis syndrome, MASS phenotype (mitral valve prolapse, aortic root diameter at upper limits of normal for body size, skin and skeletal abnormalities), Sphrintzen-Goldberg syndrome with craniosynostosis, and familial associations of thoracic aorta aneurysms and dissection.13,16
The first indications of the genetic heterogeneity of MFS came in 1994 from a study of a family of over 170 members in the south of France with a similar phenotype to classic MFS but in whom mutations in FBN1 and FBN2, the gene for fibrillin-2, were excluded. Subsequent investigation identified a second locus for MFS, located at 3p24.2-p25.7,13,18 In 2004, in individuals with a similar phenotype to MFS, mutations were found in the gene for transforming growth factor, beta receptor II (TGFBR2), mapped to the above region, providing further evidence of the disease's genetic heterogeneity. These mutations lead to MFS type 2, which is characterized by more severe involvement of the cardiovascular system, particularly aortic dissection at younger ages and with smaller aortic diameter, skeletal lesions, and mild or absent ocular involvement.7,8,10,19–23 Reports appeared in 2006 of individuals with a similar phenotype to MFS and with point mutations in the gene for transforming growth factor, beta receptor I (TGFBR1), which also came to be associated with MFS type 2.7,20,22 Some investigators have studied the possibility that the gene for transforming growth factor, beta receptor III (TGFBR3), may also be associated with MFS, but the results to date are inconclusive.7
The Universal Mutation Database has recorded 3077 mutations in FBN1, 123 in TGFBR1 and 203 in TGFBR2,24 while the Genome Aggregation Database describes 3905 mutations in FBN1, 607 in TGFBR1 and 647 in TGFBR2.25
When diagnosed early and treated appropriately, individuals with a mutation in FBN1 have a similar prognosis to those with mutations in TGFBR2. However, since TGFBR2 mutations are usually associated with less marked manifestations, these individuals are likely to be diagnosed later, often following a cardiovascular complication, and thus often have a worse prognosis.10
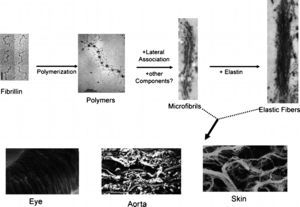
PathogenesisIt was initially thought that MFS was caused by a loss of integrity of connective tissue secondary to mutations in FBN1 that lead to abnormalities in the structure and function of microfibrils in the extracellular matrix.26–28 Microfibrils are important in the process of elastogenesis, and are particularly plentiful around the inner amorphous core of elastin, but less so inside the core. They make up the fibrillar component of elastic fibers (Figure 2).10,13,29
Schematic representation of the major steps underlying the assembly of microfibrils and elastic fibers (top) with three examples of tissue-specific networks (bottom) (adapted from Ramirez et al.30).
In the tunica media of the aorta, microfibrils associated with elastin form concentric laminae separating layers of smooth muscle cells, the resulting structure providing the aortic wall with its elasticity. They also play an important role in maintaining the aorta's mechanical stability, by interlinking the elastic laminae with smooth muscle cells and the subendothelial basement membrane.28,30 The amino acid sequences arginine-glycine-aspartate in fibrillin-1 interact with integrin alpha V beta 3, adhering to and fixing smooth muscle cells and coordinating contractile and elastic forces.31,32 Integrin alpha V beta 3 also regulates the activity of smooth muscle cells, including migration, proliferation and survival.33
Mutations in FBN1 increase the susceptibility of fibrillin-1 to proteolysis, leading to microfibril fragmentation and hence disorganization of elastic fibers in the tunica media of the aorta, a histological marker of MFS known as medial degeneration.3 This degeneration increases aortic stiffness and reduces the elasticity and distensibility of the aortic wall, alterations that promote aortic dilatation and dissection.2,34 This is followed by diffuse calcification of the tunica media, since elastic fibers are more susceptible to calcium deposition when they have lost their microfibril network. At the same time, weakened interaction between fibrillin-1 and smooth muscle cells reduces the ability of the latter to secrete molecules that provide active protection against local calcification, such as osteoprotegerin and matrix GLA protein.31,34
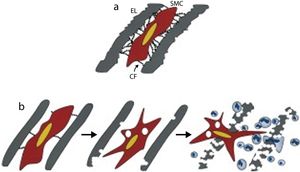
Furthermore, the loss of fibrillin-1 microfibrils leads to phenotypic alterations in smooth muscle cells, which change from a quiescent, contractile state to a proliferative state due to changes in their morphological and synthetic programming.13,30–33 This results in a loss of smooth muscle cell contractility, which normally limits the tendency of the aorta to dilate in each cardiac cycle, contributing to aortic dilatation.34 These phentotypically altered smooth muscle cells begin to secrete various components of the extracellular matrix (collagen, proteoglycans and elastin) as well as mediators of elastolysis, including metalloproteinase (MMP)-2 and MMP-9 (Figure 3).13,30,32 These enzymes induce the loss and fragmentation of elastin fibers, resulting in extensive vascular remodeling and progressive destruction of connective tissue that lead to the development of aortic aneurysms and valve lesions.31,34,35 In this vicious cycle, the fragmentation of fibrillin-1 leads to increased levels of MMPs, the elastolytic effects of which produce more fibrillin-1 fragments.34 The type of MMP that predominates appears to correlate with the stage of development of the aneurysm, with MMP-2 being expressed at higher levels in smaller aneurysms, while MMP-9 is elevated in larger or ruptured aneurysms.36 Several studies have demonstrated an imbalance between levels of MMPs and their tissue inhibitors in tissue samples from aortic aneurysms.31,34,36 Phenotypical alterations in smooth muscle cells change their state of differentiation, resulting in proliferation and migration to the tunica intima of the aorta, where rapid and disorganized ectopic accumulation of collagen and elastin takes place, leading to thickening and hyperplasia (neointima formation), a characteristic finding in the walls of aortic aneurysms in MFS patients.31,37,38
(a) Mechanism of attachment of smooth muscle cells to neighboring elastic laminae via connecting filaments (CFs) composed of fibrillin-1; (b) model of elastolysis in Marfan syndrome, showing how alterations to the synthetic program of smooth muscle cells contribute to early elastolysis, which in turn is associated with infiltration of inflammatory cells into the tunica media, precipitating structural collapse of the vessel wall (adapted from Bunton et al.31). CF: connecting filament; EL: elastic lamina; SMC: smooth muscle cell.
The degradation of fibrillin-1 and resulting deterioration in the internal and external elastic layers of the aortic wall promote macrophage chemotaxis, with infiltration of inflammatory cells into the tunica media and heightened local activity of proteolytic enzymes that intensify elastolysis. These processes contribute to the structural collapse of the aortic wall, resulting in aneurysm formation and aortic dissection.13,30,31,34,39
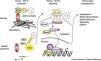
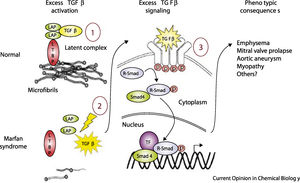
Recent studies in animal models of MFS have shown that microfibrils have other physiological functions beyond their structural role, particularly in modulating transforming growth factor beta (TGF-β) signaling.4,10,23,28,40–42 TGF-β, a cytokine secreted by various cell types in the vessel wall, is involved in the regulation of cell proliferation, migration, differentiation, and survival.8,12,42 It is secreted in the form of a large latent complex (LLC), consisting of TGF-β, a latency associated peptide (LAP), and one of three isoforms of LTBP. Immunochemical methods have demonstrated that this complex binds via the appropriate LTBP isoform to specific sites in the extracellular matrix, the fibrillin-1 domains homologous to those in the LTBP. This interaction stabilizes the latent TGF-β complex, blocking the release and activation of TGF-β.12,43–45
Mutations in FBN1 can lead to failure of matrix sequestration of the LLC and hence to activation of TGF-β.2,4,12,23,30,41–45 This free and active TGF-β interacts with TGF-β receptor 2 on the cell surface, which activates TGF-β receptor 1 by transphosphorylation. The latter receptor recruits and phosphorylates receptor-activated Smad (small mothers against decapentaplegic) proteins, which then bind to Smad4 and translocate to the nucleus, where, in combination with transcription factors, they form an active transcriptional complex that can activate or suppress numerous genes. These effects induce several of the phenotypic characteristics of MFS, including developmental emphysema, myxomatous changes of the mitral valve and mitral valve prolapse, aortic aneurysm formation and myopathy (Figure 4).4,12,42
Model of normal regulation of TGF-β by microfibrils and perturbations associated with microfibrillar deficiency in Marfan syndrome (adapted from Ramirez et al.4). LAP: latency-associated peptide; LTBP: latent transforming growth factor binding protein; p: phosphorylation; R-Smad: receptor-activated Smad protein; Smad: small mothers against decapentaplegic protein; TF: transcription factors; TGF-β: transforming growth factor beta.
Morphological and functional alterations in the atrioventricular valves correlate with increased TGF-β activity and the expression of genes it regulates, including βIGH3, EDN1 and TIMP1, that increase cell proliferation and reduce apoptosis in the valve leaflets, contributing to myxomatous alterations in the valves.46,47
Some studies implicate TFG-β in degradation of the extracellular matrix by inducing expression of MMPs, whose role in the progression of aneurysms is well known.12 MMPs, in particular MMP-2 and MMP-9, in turn activate latent TFG-β, initiating a vicious cycle.34 Active TFG-β also promotes cell proliferation and transdifferentiation of the smooth muscle cell phenotype, both seen as part of the pathogenesis of MFS.38
Identification of mutations in TGFBR1 and TGFBR2 associated with diseases that are phenotypically similar to MFS is further evidence of the contribution of excess activation of the TGF-β signaling pathway in the pathogenesis of MFS.4,7,10,12,23,28,32,41,44,48
Clinical manifestationsMFS is a multisystem disease that typically affects the cardiovascular, skeletal and ocular systems, and may also involve the central nervous system, the respiratory apparatus and the skin.5,6
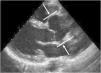
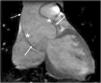
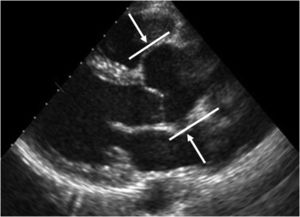
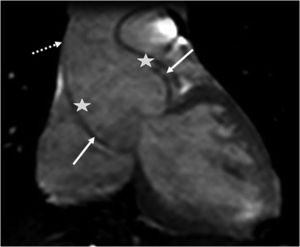
The main cardiovascular manifestation of MFS is dilatation of the aortic root and the proximal ascending aorta, with effacement of the sinotubular junction. This is found in 80% of MFS patients and can lead to aortic dissection, which is the leading cause of premature death in these patients (Figures 5 and 6).3,12,41,49–51 The ascending aorta is most severely affected due to its higher elastic fiber content and to its exposure to repeated hemodynamic stress from left ventricular ejection.2,50,52,53
MFS is found in 50% of patients with aortic dissection aged under 40 years, and in only 2% of older individuals with aortic dissection.3 Type A aortic dissection on the Stanford classification can progress in an antegrade direction to involve the aortic arch and distal segments, or retrogradely to involve the aortic valve and the coronary arteries, or can rupture into the pericardium or pleura.1,41 Risk factors for aortic dissection in MFS include diameter of upper aorta >5 cm, rapid progression of dilatation (>0.5 cm per year), family history of dissection, reduced aortic distensibility, and moderate to severe aortic regurgitation.2,41,54–56 Aortic diameter is considered the strongest predictive factor.10 Aortic dissection is extremely rare in children, irrespective of aortic size.41 Although the ascending aorta is most often affected, dilatation and dissection of the distal aorta and its branches (Stanford type B) is an important complication in MFS.3 In one study of MFS patients, dissection of the descending aorta was associated with ascending aorta dissection in 43% of cases and was isolated in 20%.57 It has been found that following elective surgery on the ascending aorta, the aortic arch and descending aorta become susceptible to aortic events, and so monitoring by imaging is important to prevent dissection in these regions.57,58 Dissection of the abdominal aorta has been observed in some MFS patients.3
Aortic regurgitation is usually a late development in MFS, secondary to dilatation of the aortic root.1,41 The risk of regurgitation correlates with aortic diameter; it is uncommon when this is <40 mm, and is almost always found with diameter >60 mm.59 Thickening, lengthening and redundancy of the mitral and tricuspid valves is also frequent, often associated with valve prolapse, with varying degrees of regurgitation.12,41,47 The mitral valve is more likely to prolapse than the tricuspid, presumably because of its location in a higher-pressure system.59
MFS is very often difficult to diagnose in childhood, since some of its clinical manifestations only become evident at older ages. Children with characteristics suggestive of MFS should undergo long-term follow-up. Most children with MFS present aortic root dilatation, mitral valve prolapse, or both, before the age of 18 years.3,60,61 An early and severe presentation frequently includes mitral valve prolapse and regurgitation, leading to dilatation, impaired left ventricular function and pulmonary hypertension. Mitral regurgitation is the leading cause of MFS-related morbidity and mortality in children, in whom mitral valve dysfunction is more common than aortic dilatation.12,41,60,62
Cardiac arrhythmias are frequent in MFS patients and are a potential cause of sudden death.62 An increased prevalence of long QT interval on the electrocardiogram has been reported.41,63
Pulmonary artery dilatation is common in individuals with MFS, more pronounced at the arterial root. Pulmonary artery dissection is rare, but may be becoming more important with increasing longevity of MFS patients.6,52,64 Pulmonary regurgitation has also been reported.50
The most obvious skeletal abnormality in MFS, and one that frequently raises suspicion of the diagnosis, is disproportionate growth of the limb bones, resulting in tall stature, reduced upper to lower segment ratio, and an arm-span to height ratio of at least 1.05. Excessive longitudinal growth of the ribs leads to thoracic abnormalities (pectus escavatum or pectus carinatum). The presence of arachnodactyly is a subjective judgment based on signs on physical examination.12,41 Thoracolumbar scoliosis affects around 60% of MFS patients,65 while acetabular protrusion is usually asymptomatic in young adults.41 Pes planus is relatively common, while pes cavus is less so. Camptodactyly is a common finding, especially in children with rapid and severe disease progression. Various facial characteristics are typically present, including dolichocephaly, high-arched palate, dental crowding, retrognathia or micrognathia, malar flattening, and down-slanting palpebral fissures.6,41
The most frequent ocular manifestation of MFS is lens subluxation (ectopia lentis), found in around 60% of cases. Other findings include early and severe myopia, retinal detachment, early-onset cataract or glaucoma, flat cornea, and hypoplastic iris.12,41
Other systemic alterations include dural ectasia in 60-90% of cases, with swelling of the dural sac in the lumbar and sacral spine.12 Pulmonary involvement may result from severe deformation of the sternum or vertebral column, leading to a restrictive ventilatory defect. Finally, skin striae and inguinal hernias are common both at birth and in adolescence, the latter with a high risk of recurrence after surgery.41
DiagnosisGiven the progressive and potentially fatal character of the clinical manifestations of MFS, early diagnosis is of the utmost importance.66
Diagnosis is often difficult due to the disease's considerable interfamilial and intrafamilial phenotypical variability, the age-dependent nature of some of its clinical manifestations, the high proportion of spontaneous mutations, and the similarity of its presentation to several other connective tissue diseases.4,65–68 The 1996 Ghent nosology included major and minor manifestations involving different systems and family and genetic history. However, these criteria had some limitations, and so a revised version was published in 2010. These new criteria were designed to enable an early diagnosis, to avoid ambiguous diagnoses, and to facilitate follow-up and monitoring of patients diagnosed with the disease (Tables 1 and 2).5,6
Revised Ghent criteria for diagnosis of Marfan syndrome and related conditions.
| In the absence of family history:Ao (Z≥2) and EL = MFSaAo (Z≥2) and FBN1 mutation = MFSAo (Z≥2) and Sist (≥7 points) = MFSaEL and FBN1 mutation with known aortic aneurysm = MFSEL with or without Syst and with FBN1 mutation not previously associated with aortic root aneurysm/dissection or no FBN1 mutation = ELSAo (Z<2) and Syst (≥5 with at least one skeletal feature) without EL = MASSMVP and Ao (Z<2) and Syst (<5) without EL = MVPSIn the presence of family history:EL and family history of MFS (as defined above) = MFSSyst (≥7 points) and family history of MFS (as defined above) = MFSaAo (Z≥2 above 20 years old, ≥3 below 20 years) and family history of MFS (as defined above) = MFSaa Caveat: without discriminating features of Sphrintzen-Goldberg syndrome, Loeys-Dietz syndrome or the vascular form of Ehlers-Danlos syndrome and after TGFBR1/2, collagen biochemistry, and COL3A1 testing if indicated. Other conditions/genes will emerge with time. |
| Ao: aortic diameter at the sinuses of Valsalva above indicated Z-score or aortic root dissection; COL3A1: gene for type III collagen; EL: ectopia lentis; ELS: ectopia lentis syndrome; FBN1: gene for fibrillin-1; MASS: MASS phenotype (myopia, mitral valve prolapse, borderline aortic root dilatation (Z<2), skin striae, skeletal findings); MFS: Marfan syndrome; MVP: mitral valve prolapse; MVPS: mitral valve prolapse syndrome; Syst: systemic score (see Table 2); TGFBR1/2: genes for transforming growth factor, beta receptor I/2; Z: Z-score.Adapted from Loeys et al.6 with permission from BMJ Publishing Group Limited. |
Scoring of systemic features in the revised Ghent nosology.
| ▶Wrist AND thumb sign – 3 (wrist OR thumb sign – 1)▶Pectus carinatum – 2 (pectus escavatum or chest asymmetry – 1)▶Hindfoot deformity – 2 (plain pes planus -1)▶Pneumothorax – 2▶Dural ectasia – 2▶Acetabular protrusion – 2▶Reduced upper/lower segment ratio AND increased arm/height AND no severe scoliosis – 1▶Scoliosis or thoracolumbar kyphosis – 1▶Reduced elbow extension – 1▶Facial features (3/5) – 1 (dolichocephaly, enophthalmos, down-slanting palpebral fissures, malar hypoplasia, retrognathia)▶Skin striae – 1▶Myopia >3 diopters – 1▶Mitral valve prolapse (all types) – 1Maximum total: 20 points; score ≥7 indicates systemic involvement. |
Adapted from Loeys et al.6 with permission from BMJ Publishing Group Limited.
The revised nosology gives greater weight to aortic root aneurysm, aortic dissection, and ectopia lentis, with other clinical manifestations becoming part of the systemic score calculation.6,69
Initial assessment of a suspected case of MFS should include personal and family history, complete physical examination, and tests to determine the presence of diagnostic criteria.
Cardiovascular study usually entails transthoracic echocardiography to determine the maximum diameter of the aortic root at the sinus of Valsalva in at least three transthoracic images. The mean diameter should be corrected for age and body surface area (BSA) and interpreted according to the Z-score; Roman et al. created a nomogram for aortic root diameter in relation to BSA and age.6,70,71 If transthoracic echocardiography does not allow precise visualization of the proximal aorta, transesophageal echocardiography, computed tomography or magnetic resonance imaging should be performed, using the same nomogram to obtain correct diameter measurements.6 The latter imaging modalities are also indicated for study of the distal aorta. Echocardiography is also valuable for assessment of morphological and functional alterations of the mitral and tricuspid valves.
Assessment of the ocular, skeletal and other systems is carried out using appropriate methods.
Differential diagnosis in MFS includes various diseases associated with aortic aneurysms (Loeys-Dietz syndrome, bicuspid aortic valve, familial thoracic aortic aneurysm syndrome, Ehlers-Danlos syndrome, arterial tortuosity syndrome), ectopia lentis (ectopia lentis syndrome, Weill-Marchesani syndrome, homocystinuria, Stickler syndrome), or similar systemic manifestations to MFS (Sphrintzen-Goldberg syndrome, congenital contractural arachnodactyly, Loeys-Dietz syndrome, MASS phenotype, and mitral valve prolapse syndrome).6
First-degree relatives of MFS patients should undergo a thorough physical examination, ophthalmological assessment, and echocardiographic study of the aortic root and mitral valve. If one or more relatives of a patient with known thoracic aortic aneurysm and/or dissection are found to have thoracic aortic dilatation, aneurysm, or dissection, then imaging of second-degree relatives is reasonable.
If a mutant gene (FBN1, TGFBR1, TGFBR2) associated with aortic aneurysm and/or dissection is identified in an MFS patient, first-degree relatives should undergo counseling and genetic testing, and only relatives with the mutation should undergo aortic imaging.3,62,72
TreatmentMedicalThe standard of medical care for prevention of aortic complications in MFS is beta-blockade.6,72 The beneficial effects of beta-blockers are attributed to reductions in heart rate and left ventricular ejection fraction (reducing hemodynamic stress on the aortic wall), in aortic stiffness (and hence aortic root dilatation), and in risk of aortic dissection and other cardiovascular complications including aortic regurgitation and need for surgery, thereby improving survival.3,40,61,65,73–76 The beta-blocker should be titrated to aim for a heart rate after submaximal exercise of <100 bpm in individuals over five years of age.6 These drugs should be prescribed for all MFS patients, including those with aortic root diameter <4 cm and children.6,72
Experiments in mouse models of MFS show that losartan, an angiotensin II type 1 receptor blocker, can prevent progression of aortic dilatation.6,77 Similarly promising results were achieved in a pilot study on children with severe MFS.6,78 Its benefit appears to be due to its antihypertensive action and ability to reduce plasma TGF-β levels, block the transcription of genes that respond to TGF-β, and decrease levels of intracellular mediators of the TGF-β signaling cascade such as Smad2. In addition, losartan does not affect the angiotensin II type 2 receptor, which unlike the type 1 receptor has anti-inflammatory and antiproliferative effects that are beneficial for homeostasis in the aortic wall.77–79 A recent meta-analysis concluded that there were no differences in the effect of losartan and atenolol on rates of dilatation of the aortic root and ascending aorta and on aortic complications, and found no advantage of one agent over the other.80 A study in a mostly pediatric population with MFS demonstrated that therapy with a beta-blocker and losartan simultaneously was safe and was more effective at reducing or even reversing aortic root dilatation than beta-blocker therapy alone. However, there is still a lack of evidence for this beneficial effect.81,82
One area of research is inhibitors of MMPs, with the aim of stabilizing the progression of aneurysmal disease. Doxycycline is a non-specific MMP inhibitor, via mechanisms unrelated to its antibiotic action; its maximum inhibition is seen against MMP-2 and MMP-9. In a study of animal MFS models, doxycycline was more effective than atenolol in preventing aneurysm formation, by preserving elastic fiber integrity, normalizing vasomotor function, and reducing TGF-β activation.83
Patients with MFS are advised to avoid cigarette smoking and to have their blood pressure monitored, keeping levels below 120/80 mmHg.3 They should not take part in sports involving intense physical exertion or with likelihood of significant bodily contact. However, it is reasonable for athletes with MFS to participate in low and moderate static/low dynamic competitive sports, if they do not have any of the following: aortic root dilatation (Z-score>2, or aortic diameter >40 mm, or >2 standard deviations from the mean relative to BSA in children or adolescents <15 years old); moderate to severe mitral regurgitation; left ventricular systolic dysfunction (ejection fraction <40%); or family history of aortic dissection at an aortic diameter <50 mm.84
SurgicalType A aortic dissection in MFS is an indication for emergent aortic surgery, which is associated with operative mortality of 20% and 10-year survival of 50-70%. On the other hand, the risk of death associated with prophylactic aortic surgery is only 1-2%, and survival of patients undergoing elective surgery is better than in those treated by emergent surgery, approaching that of the general population. These data highlight the importance of prophylactic aortic surgery for patients with aortic root dilatation.6,68,85–88
Prophylactic aortic surgery for MFS patients was pioneered by Bentall and Bono in 1968.89 It consists of replacement of the aortic root, aortic valve and ascending aorta with a mechanical composite graft (combined vascular and valve) and reimplantation of the coronary arteries in the vascular graft. The procedure's safety, reproducibility, and long-term durability are well documented.61,90 Subsequent developments include the remodeling procedure of Yacoub in 1979 and the reimplantation technique of David in 1988, in both of which the aortic root is replaced but the native aortic valve is spared. Recent studies indicate that these surgical procedures are associated with reduced operative risk and a low rate of subsequent aortic valve replacement, as well as excellent long-term survival, when performed on a structurally normal aortic valve.90–94 According to the most recent guidelines, prophylactic surgery should be performed in MFS patients who have aortic root aneurysm and a maximum aortic diameter ≥50 mm, using the David reimplantation technique or the Yacoub procedure or, if these are not technically feasible, the Bentall procedure. Surgery should also be considered in patients with an ascending aortic diameter of ≥45 mm and any of the following risk factors: family history of dissection, size increase >3 mm/year (in repeated examinations using the same technique, at the same point of the aorta, and confirmed by another technique), severe aortic or mitral regurgitation, or desire for pregnancy.95
Although conventional surgery remains the treatment of choice for MFS patients with thoracic aorta disease, thoracic endovascular aortic repair (TEVAR) offers a less invasive alternative. However, according to the European position statement, TEVAR is not recommended as first-line therapy in patients with connective tissue disease except as a bail-out procedure or bridge to definitive open surgical therapy, or following prior aortic repair when the graft can be implanted over the previously placed prosthesis.96 The latest European guidelines on the diagnosis and treatment of aortic diseases recommend that in MFS, surgery should be preferred over TEVAR, except in emergency situations in order to achieve initial stabilization as a bridge to definitive surgical therapy.95
Follow-upAn echocardiogram is recommended at initial diagnosis and six months thereafter to determine the rate of enlargement of the aorta. Annual imaging is recommended if stability of aortic diameter is documented. More frequent imaging should be performed if the aortic diameter is approaching a surgical threshold (≥4.5 cm in adults; less well defined in children) or shows rapid change (≥0.5 cm/year) or if there are concerns regarding heart or valve function. Individuals under age 20 years with systemic findings suggestive of MFS but no cardiovascular involvement should have annual echocardiograms due to the potential for rapid evolution of the phenotype. Adults with repeatedly normal aortic root measurements can be seen at intervals of 2-3 years.6,72
Patients with MFS can develop aortic dilatation or dissection at segments more distant from the aortic root. The frequency of this finding (particularly at the proximal descending thoracic aorta and in the abdomen) appears to be increasing with prolonged survival due to improved management of disease at the aortic root. Although this is not recommended in the guidelines, intermittent imaging studies can be performed for early detection of distal aortic disease.6 Periodic imaging of the entire aorta is recommended in cases of dilatation or dissection of the distal aorta, and also annually following surgical repair of the aortic root, especially in cases of rapidly progressing dilatation and/or aortic diameter >4.5 cm.72
PrognosisThe cardiac complications of MFS, particularly aortic dilatation, dissection and rupture and involvement of the aortic and mitral valves, result in a significantly reduced life expectancy. This poor survival was demonstrated in a series of 257 patients with MFS published in 1972, in whom the mean age at death for the 72 deceased patients was 32 years. Cardiac problems led to 52 of the 56 deaths of known cause, with aortic dilatation and its complications accounting for 80 per cent of these. Congestive heart failure, myocardial infarction and infective endocarditis were other cardiac causes of death.56,88,97
However, life expectancy in MFS has improved significantly since the introduction of beta-blocker therapy and elective surgical repair of the aorta, rising to more than 72 years according to recent series.3,61,72,98,99
ConclusionsMFS is a rare genetic disease with high morbidity. Currently available diagnostic and therapeutic methods have enabled a better understanding of its pathogenesis and have also significantly improved the prognosis of the disease.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Coelho SG, Almeida AG. Síndrome de Marfan revisitada – da genética à clínica. Rev Port Cardiol. 2020;39:215–226.