The index of microcirculatory resistance (IMR) provides quantitative, invasive, and real-time assessment of coronary microcirculation status.
AimsThe primary aim of this study was to validate the assessment of IMR in a large animal model, and the secondary aim was to compare two doses of intracoronary papaverine, 5 and 10 mg, for induction of maximal hyperemia and its evolution over time.
MethodsMeasurements of IMR were performed in eight pigs. Mean distal pressure (Pd) and mean transit time (Tmn) were measured at rest and at maximal hyperemia induced with intracoronary papaverine, 5 and 10 mg, and after 2, 5, 8 and 10 minutes. Disruption of the microcirculation was achieved by selective injection of 40-μm microspheres via a microcatheter in the left anterior descending artery.
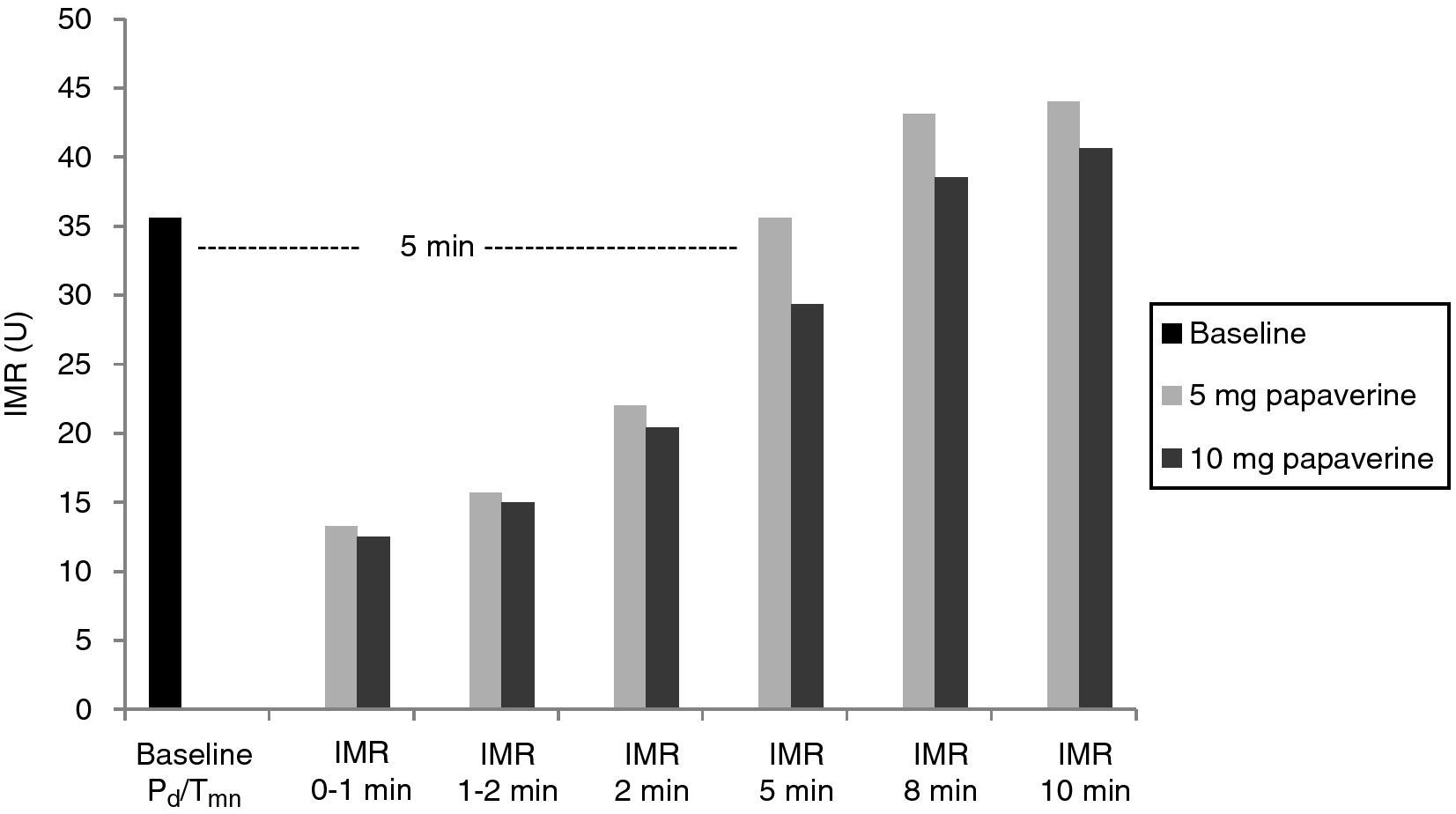
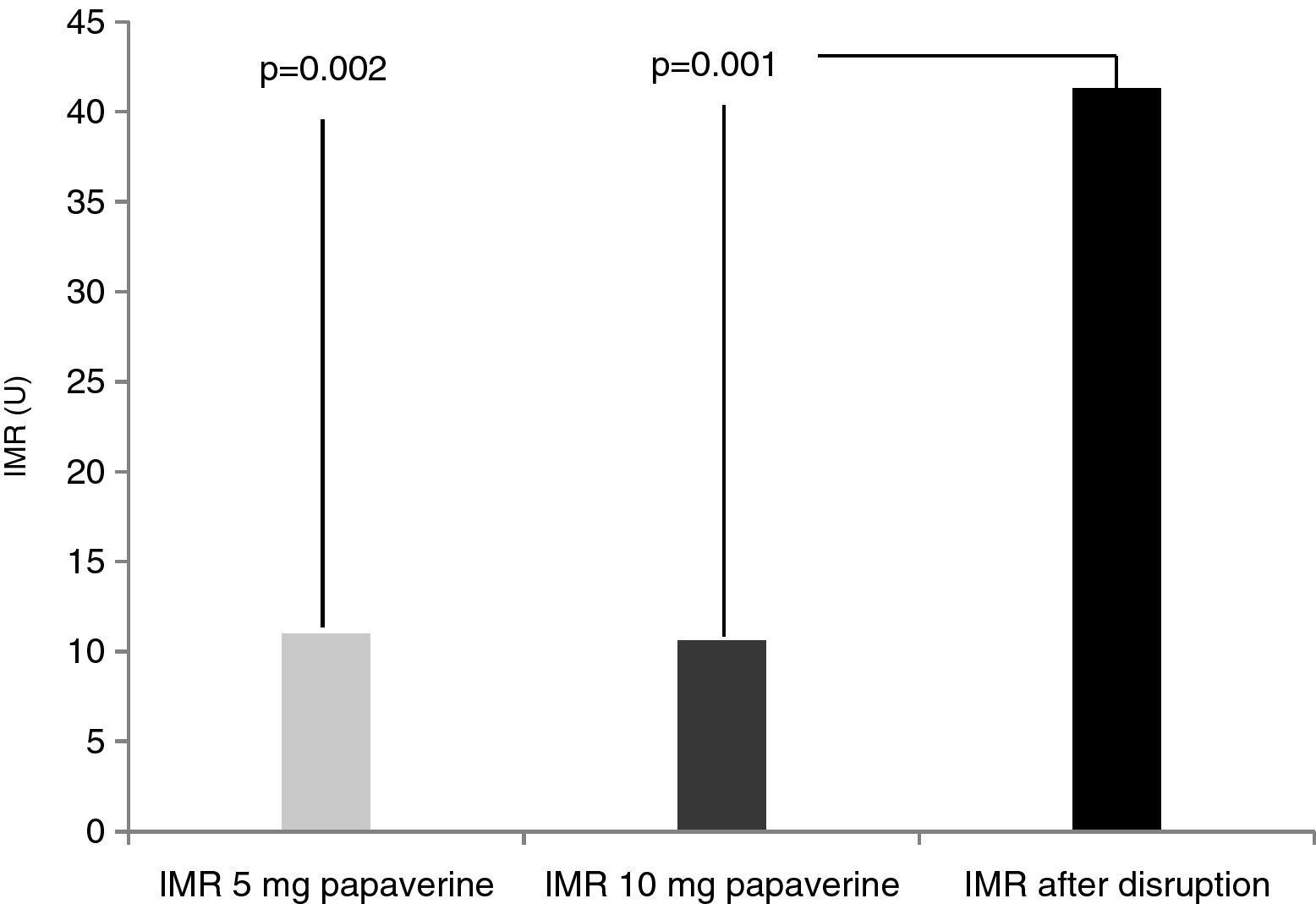
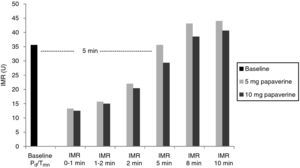
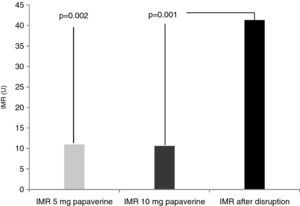
ResultsIn each animal 14 IMR measurements were made. There were no differences between the two doses of papaverine regarding Pd response and IMR values – 11±4.5 U with 5 mg and 10.6±3 U with 10 mg (p=0.612). The evolution of IMR over time was also similar with the two doses, with significant differences from resting values disappearing five minutes after intracoronary papaverine administration. IMR increased with disrupted microcirculation in all animals (41±16 U, p=0.001).
ConclusionsIMR provides invasive and real-time assessment of coronary microcirculation. Disruption of the microvascular bed is associated with a significant increase in IMR. A 5-mg dose of intracoronary papaverine is as effective as a 10-mg dose in inducing maximal hyperemia. Five minutes after papaverine administration there is no significant difference from resting hemodynamic status.
O índice de resistência da microcirculação (IRM) permite o estudo quantitativo, invasivo e em tempo real do estado da microcirculação coronária. O objetivo principal foi a validação da técnica de determinação do IRM num modelo animal de grande porte. O objetivo secundário foi a comparação de duas doses de papaverina, 5 e 10 mg, para indução de hiperémia máxima e quanto à sua evolução temporal. Foram estudados oito porcos. Avaliou-se a Pd e o Tmn em condições basais e com hiperemia máxima induzida pela injeção intracoronária de 5 ou 10 mg de papaverina e sucessivamente aos dois, cinco, oito e dez minutos. Para o compromisso da microcirculação foram injetadas microesferas de 40 μm de diâmetro. Em cada animal foram realizadas 14 determinações de IRM. Não se observaram diferenças significativas entre as duas doses de papaverina quanto às repostas da Pd e ao valor de IRM obtido, 11±4.5 U com 5 mg de papaverina e de 10,6±3 U com a dose de 10 mg (p=0,612). A evolução temporal dos valores de IRM foi semelhante, deixando de haver diferenças relativamente ao valor basal após cinco minutos. Com a injeção das microesferas houve uma elevação significativa do IRM (41±16 U, p=0,001). O IRM avalia em tempo real a resistência da microcirculação coronária. A administração intracoronária de 5 mg de papaverina é tão eficaz como a de 10 mg para a indução de hiperemia máxima, com o retorno às condições basais a ocorrer cinco minutos após a sua administração.
Advances in sensor technology mean that coronary flow pressure and velocity (by Doppler or thermodilution) can now be measured using a single guidewire, enabling hemodynamic study of the entire coronary circulation.1,2 Two pathophysiological resistances contribute to various forms of heart disease: epicardial stenosis and microvascular dysfunction. There are well-established and widely used parameters for the former such as fractional flow reserve, but invasive real-time study of the microcirculation that is feasible in clinical practice is still in its early stages.3
Coronary flow reserve (CFR) is the maximum increase in blood flow through the coronary arteries above the normal resting volume, the maximum flow being achieved through pharmacologically induced hyperemia. The disadvantages of CFR are that it is dependent on baseline hemodynamic conditions, and that the information it provides cannot differentiate between the epicardial circulation and the microcirculation.4,5 These drawbacks can be overcome by use of the index of microcirculatory resistance (IMR),6 which is measured at maximum flow, and is defined as the distal coronary pressure (Pd) at maximal hyperemia divided by the inverse of the mean transit time (Tmn). Tmn is determined by thermodilution; after a series of intracoronary injections of 3 ml of room-temperature saline, which is detected by the shaft of the pressure wire acting as a proximal thermistor, the saline flows through the coronary artery to the distal thermistor and the mean transit time between the two sensors is calculated. IMR is highly reproducible and independent of heart rate, blood pressure and contractility.7
Large animal models are essential for preclinical study of the microcirculation. Pigs are the most suitable since their cardiac vascularization, coronary anatomy, and poor subendocardial to epicardial collateral network are similar to humans.8,9
A key step in the assessment of IMR is induction of maximal hyperemia. This is achieved by vasodilation of the entire coronary tree using drugs with vasodilatory effects on small vessels, of which intravenous adenosine and intracoronary papaverine are the most effective.10 However, different doses of these drugs have been used in animal studies and it has not been established what dosage is best or whether a dose effect needs to be taken into consideration when interpreting the results.
The complexity of animal research, as well as of the techniques for determining IMR, means that the models must be validated before they can be used to study the pathophysiology of heart disease or to assess the effects of a particular therapy on the microcirculation.
AimsThe primary aim of this study was to validate the assessment of IMR in a large animal model, and the secondary aim was to compare two doses of intracoronary papaverine, 5 and 10 mg, for induction of maximal hyperemia and its evolution over time.
MethodsMeasurements of IMR were performed in eight pigs, crosses between Duroc males and F1 Large White × Landrace females, live weight 25–40 kg (32±5). The study was approved by the Institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine of the University of Lisbon. The animals used in this study were not sacrificed; during the 24-hour recovery period they were given analgesics (2 mg/kg carprofen [Rimadyl, Pfizer]) and antibiotics (15 mg/kg amoxicillin [Clamoxyl LA, Pfizer]), and showed no signs of suffering.
All the animals were maintained in fasting conditions for the 12 hours before the procedure. The pre-anesthetic medication used was 2 mg/kg azaperone (Stresnil®), 1 mg atropine and, after 15 min, 20 mg/kg ketamine chlorhydrate (Imalgene 1000®). In the catheterization laboratory, a 22G venous catheter (Abbocath®) was placed in the marginal vein of the ear, through which 6 mg/kg of 5% sodium thiopental (Tiopental Braun®) was administered. After confirmation of loss of the swallowing reflex, the animals were intubated and placed under controlled ventilation with a tidal volume of 10 ml/kg. Anesthesia was maintained with 2% isoflurane (Isoflo®, Abbott Laboratories) in constant O2 flow. Continuous intravenous 9% saline was administered until the end of the procedure and body temperature was maintained with a thermal blanket.
Hemodynamic and ECG monitoring was performed throughout the procedure, as well as 12-lead ECG at the beginning and end. Following the placement of 6F introducers in the right femoral artery and vein, 200 U/kg heparin was administered. Arterial pressure in the aorta and end-diastolic pressure were measured using a pigtail catheter (Cordis). Selective catheterization of the left coronary artery was performed using a 6F 0.75-cm Amplatz Left guiding catheter, followed by intracoronary injection of 0.5–1.0 mg isosorbide dinitrate (Isoket®, Schwarz Pharma AG) and baseline angiography.
A 0.014″ PressureWire™ Certus guidewire (St. Jude Medical) was positioned with the sensor at the junction of the mid and distal thirds of the left anterior descending (LAD) artery after equalization of pressures with the sensor positioned just below the tip of the catheter. If difficulties were experienced with navigating in the LAD, a BMW guidewire (Abbott Vascular) was used, the wires being switched by means of a FineCross™ microcatheter (Terumo Interventional Systems).
A RadiAnalyzer™ Xpress system (St. Jude Medical) and associated software was used to determine Pd and Tmn in the LAD. After a series of intracoronary injections of 3 ml of room-temperature saline via the guidewire, the mean of the three measurements that were closest in value and had similar temperature-time curves were used to calculate Tmn. IMR is calculated by dividing coronary Pd by the inverse of Tmn. In view of the aims of this study all measurements were considered to be IMR, but only that taken at maximal hyperemia was taken to be the true IMR.
Following measurement of Pd and Tmn in resting conditions and keeping the guidewire in the same position, IMR was measured at maximal hyperemia induced by intracoronary injection of 5 mg papaverine, after confirmation of a fall in distal pressure during the first minute, and at 2, 5, 8 and 10 minutes. Following a 10-minute interval for metabolization of the previously administered papaverine, 10 mg papaverine was injected and the sequence of measurements was repeated.
Disruption of the microcirculation was achieved by selective injection of 0.25 ml of a solution containing approximately 30×106 40-μm Embozene® microspheres (CeloNova BioSciences) suspended in contrast via a microcatheter with its tip placed in the LAD distal to the first diagonal and proximal to the pressure wire microsensor, which was maintained in the same position. IMR was determined after five minutes, at maximal hyperemia induced by the intracoronary injection of 10 mg papaverine during the first minute and after confirmation of a fall in distal pressure.
Statistical analysisNormal distribution of the data was tested using the Kolmogorov-Smirnov test with the Lilliefors correction and using the Shapiro Wilk test for small samples. Categorical variables are expressed as counts and percentages of the total, while continuous variables are expressed as means ± standard deviation. The Student's t test for paired samples was used to compare the differences between baseline values, after 5 and 10 mg of papaverine, and after microsphere injection. The statistical analysis was performed using SPSS version 20.
ResultsMean resting hemodynamic values were: heart rate 92±10 bpm, mean arterial pressure 48±5 mmHg and left ventricular end-diastolic pressure 6±1.4 mmHg. All the animals remained hemodynamically and electrically stable after administration of papaverine. ST-segment elevation was seen in V1–V3 in all animals after disruption of microcirculation but no ventricular arrhythmias or hemodynamic instability occurred.
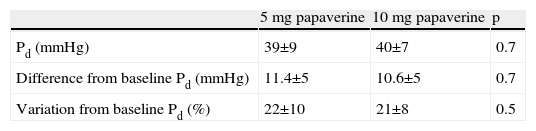
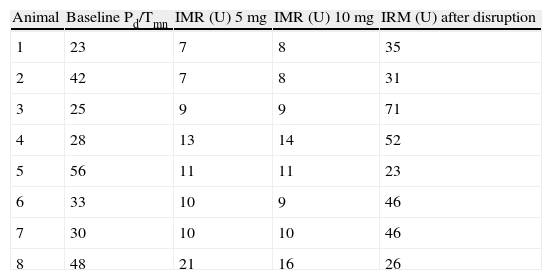
A total of 112 measurements of IMR were made, 14 in each animal. The variation in Tmn between the three measurements considered for calculation of IMR at maximal hyperemia was 10%. Mean baseline Pd was 51±9 mmHg. Table 1 presents the values of Pd, which reflect maximal hyperemia after injection of papaverine; there were no differences between the two doses. The values of IMR for each animal are shown in Table 2; mean true IMR was 11±4.5 U with 5 mg and 10.6±3 U with 10 mg papaverine (p=0.612).
Ratio of mean distal pressure and the inverse of mean transit time in resting conditions, after maximal hyperemia with 5 and 10 mg of intracoronary papaverine and after disruption of microcirculation with microsphere injection.
| Animal | Baseline Pd/Tmn | IMR (U) 5 mg | IMR (U) 10 mg | IRM (U) after disruption |
| 1 | 23 | 7 | 8 | 35 |
| 2 | 42 | 7 | 8 | 31 |
| 3 | 25 | 9 | 9 | 71 |
| 4 | 28 | 13 | 14 | 52 |
| 5 | 56 | 11 | 11 | 23 |
| 6 | 33 | 10 | 9 | 46 |
| 7 | 30 | 10 | 10 | 46 |
| 8 | 48 | 21 | 16 | 26 |
5 mg: after maximal hyperemia with 5 mg of intracoronary papaverine; 10 mg: after maximal hyperemia with 10 mg of intracoronary papaverine; IMR: index of microcirculatory resistance; Pd: distal pressure; Tmn: mean transit time.
With both doses of papaverine, true IRM was observed in the first minute in five animals, after one minute in two and after two minutes in one. The evolution of IMR over time was similar with the two doses (Figure 1), with significant differences from resting values disappearing five minutes after intracoronary papaverine administration (p=1.00 for 5 mg and p=0.98 for 10 mg).
Disrupted microcirculation following microsphere injection led to a significant increase in IMR in all animals, to a mean of 41.3±16 U (Figure 2) (p=0.002 for 5 mg of papaverine and p=0.001 for 10 mg). The mean rise in true IMR with the 10-mg dose was 310±190%.
DiscussionThere are several reasons for the increasing interest in the cardiac microcirculation. Small vessels are an important stumbling-block to percutaneous revascularization techniques, as shown by coronary no-reflow or persistence of myocardial ischemia even after intervention in the epicardial vessels, particularly in the context of myocardial infarction.11 The microcirculation is also the site of pathophysiological processes in various types of heart disease, from cardiac syndrome X12 to the poorly-defined stress cardiomyopathy (Takotsubo syndrome).13 At the same time, novel therapies being studied for heart disease, such as gene and cell therapy, affect the small vessels by stimulating angiogenesis or by requiring administration of cells via an intracoronary route.14,15
It is thus important to establish a preclinical model of the microcirculation that can be used to evaluate the effects and limitations of current and future therapies. Porcine cardiac vascularization is the closest to that of humans, and the coronary anatomy is similar in morphology and size, right down to the diameter of capillaries16; the pig is moreover a domesticated species that is readily available and easy to handle. These advantages have made the pig the model of choice for preclinical study of the cardiac microcirculation.
Microcirculatory resistance is assessed during maximal dilatation of the coronary tree, which is achieved pharmacologically by administering microcirculatory vasodilators and reflects the maximum capacity for increasing coronary flow when all the available circulatory network is recruited. Although adenosine is the most widely used drug for this purpose in clinical practice due to its safety, intracoronary papaverine is preferred in preclinical studies because it is more effective than adenosine,17 which may have limited vasodilatory capacity in large animals.18
The dose of papaverine used in different studies varies between 5 and 20 mg, the latter being the usual dose in humans, but it has not been established what dosage is best or whether different doses have significantly different effects. In our study, the lower dose (5 mg) had a similar effect to that of the higher dose of 10 mg on distal pressure fall and IMR values. The higher dose was equally safe, with no associated hemodynamic or electrical instability. Since accurate determination of IMR depends on induction of maximal hyperemia and hence on administration of a sufficient quantity of the drug, which in turn can be influenced by the selectivity or stability of the catheter itself, it is reasonable to suppose that the 10-mg dose is more likely to ensure uniform results and is thus the one to be used.
IMR was validated by Fearon et al.6 in a porcine model in which hyperemia was induced with 20 mg intracoronary papaverine in nine animals. The mean IMR was 16.9±6.5 U, slightly higher than in our study, which may be related to manipulation of the LAD during surgery in their study. These values are lower than the 21 U reported in humans without coronary disease, which is presumably partly due to extrapolation of results from porcine models to humans.16 It is also important to correct IMR using coronary wedge pressure when there is large vessel disease and collateral circulation,19 which was not the case in our animal model.
Although IMR has undoubted advantages, accurate values can only be obtained by using a consistent methodology that ensures correct calibration and placement of the guidewires, effective induction of maximal hyperemia, maintenance of saline for intracoronary injection at room temperature, and careful interpretation of time-temperature curves during maximum flow. IMR has only been in use for a short time and by a small number of investigators, and consequently not all of these methodological aspects have been fully explored or defined.
The evolution over time of hemodynamic variables following papaverine administration has important practical implications. IMR is useful to examine the immediate effect of various therapies on the microcirculation, but it is also important to know at what point baseline hemodynamic conditions return. In our study, significant differences disappeared five minutes after both doses of intracoronary papaverine. It is therefore advisable that any intervention designed for baseline conditions should be performed after this period. Our results also indicate interindividual variability in the time to maximal hyperemia, with most but not all animals showing this response in the first minute. This variability must be taken into account and several series of Tmn measurements should be performed until a nadir is reached that corresponds to the true IMR; if this is not done IMR may be overestimated.
The mean diameter of coronary capillaries is 10 μm. Intracoronary injection of 40-μm microspheres thus disrupts the microcirculation in a predictable manner by obstructing circulation downstream of small arterioles. We used a quantity of microspheres that maintains coronary flow, avoiding the no-reflow phenomenon that in our experience occurs at higher doses. The consistent and significant increase in IMR following disruption of the microcirculation while epicardial flow was not obstructed is a clear indication of the value of IMR in the study of the coronary microcirculation.
Measurement of IMR is a complex technique that needs to be perfected over time. Certain steps should be taken to minimize variations in measurements. As well as obvious factors such as selectivity of catheterization, careful pressure equalization, constant rate of saline injection, correct positioning of the pressure wire and care in the induction and timing of maximal hyperemia, in our experience there are other factors that can affect or invalidate measurement of IMR. The epicardial arteries of pigs of the body weight generally used in research are smaller than humans’ and thus harder to navigate with the pressure wire, the floppy tip of which frequently bends, resulting in falsely high readings. Even when the catheter is straight, its tip can become lodged against a small vessel, causing the wire to bend during systole, increasing variation in the time-temperature curves obtained after saline injection and distorting measurement of IMR. The pressure wire must be kept straight and free within the coronary artery, and to this end our group now use a microcatheter introduced beforehand into the LAD with a standard wire such as BMW, ensuring faster and more effective placement of the pressure wire, the tip of which is thereby protected from excessive bending. Publication of detailed procedures and solutions to such problems encountered in studies using IRM are essential to improvements in the technique and should be encouraged.
ConclusionDetermination of IMR in a porcine model enables assessment of the status of the coronary microcirculation, disruption of which is associated with significant increases in the index. A 5-mg dose of intracoronary papaverine is as effective as a 10-mg dose in inducing maximal hyperemia. Despite interindividual differences in the time before maximal hyperemia, five minutes after papaverine administration there is no significant difference from resting hemodynamic status.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fiarresga A, Selas M, Oliveira E, et al. O índice de resistência microcirculação para o estudo invasivo da microcirculação coronária – descrição e validação de um modelo animal. Rev Port Cardiol. 2014;33:207–212.