Hypertension complicates 6–8% of pregnancies and includes the following four conditions: hypertension preceding pregnancy or documented before the 20th week of gestation; pre-eclampsia (PE)/eclampsia; chronic hypertension with superimposed pre-eclampsia; and gestational hypertension. The latter is defined as a significant rise in blood pressure after the 20th week of pregnancy in previously normotensive women, to over 140/90mmHg. When blood pressure remains above 160/110mmHg, it is considered severe. PE is defined as the presence of proteinuria (≥300mg/24hours) in pregnant women with hypertension. The hypertensive syndromes of pregnancy are among the leading causes of maternal and fetal morbidity and mortality and anti-hypertensive treatment is part of the therapeutic arsenal used to prevent serious complications. Although the role of utero-placental insufficiency due to deficient migration of trophoblasts to the spiral arteries is universally accepted, the pathophysiology of PE remains largely unknown and is the subject of debate. No effective ways of predicting or preventing PE have been found, which highlights the need for further research in this field. This review aims primarily to evaluate recent advances in our understanding of the pathophysiology of gestational hypertension and especially PE, and new ways of predicting PE. Additionally, we present a brief review on the diagnosis, prevention and treatment of PE.
A Hipertensão Arterial (HTA) na gravidez complica 6 a 8% das gestações e inclui 4 principais formas de apresentação: HTA crónica, que antecede a gravidez ou é documentada antes das 20 semanas de gestação, pré-eclampsia (PE)/eclampsia, HTA crónica com PE sobreposta e HTA gestacional. A HTA gestacional define-se como uma elevação significativa da pressão arterial após as 20 semanas de gestação em gestantes previamente normotensas, atingindo valores superiores a 140/90mmHg. Quando os valores de pressão arterial se mantêm acima de 160/110mmHg de forma sustentada, é considerada grave. A pré-eclampsia (PE) define-se pela presença de proteinúria (≥300mg/24horas) em gestante com HTA. As síndromes hipertensivas da gravidez encontram-se entre as principais causas de morbimortalidade materno-fetal, sendo que a terapêutica anti-hipertensora faz parte do arsenal terapêutico utilizado na prevenção das suas graves complicações. Apesar do papel da insuficiência útero-placentária por deficiente migração dos trofoblastos para as artérias espiraladas ser universalmente aceite, a fisiopatologia da PE permanece ainda parcialmente uma incógnita e reúne alguma controvérsia. Historicamente, não são conhecidas formas suficientemente eficazes de predição e prevenção da PE, pelo que a investigação nesta área assume particular importância. Esta revisão visa primariamente avaliar os avanços científicos mais recentes nas áreas da fisiopatologia da HTA gestacional e, em particular, da PE e das novas formas de predição desta patologia. Concomitantemente, apresentamos informação sumária recente acerca do diagnóstico, prevenção e tratamento anti-hipertensor na PE.
The hypertensive syndromes of pregnancy are the leading cause of maternal and fetal morbidity and mortality in the developed world,1,2 occurring in around 8% of pregnancies. There have been numerous studies aimed at clarifying the variable presentation and systemic nature of pre-eclampsia (PE) and other hypertensive disorders of pregnancy.3,4 However, there is some disagreement in the literature concerning antihypertensive treatment in pregnant women at risk, particularly the pharmacology, efficacy and safety of the available drugs, the best methods for identifying high-risk groups, and prediction and prevention of complications.
Forty per cent of pregnant women who develop eclampsia do not present hypertension or proteinuria in the previous week. This highlights the need for preventive measures.5
Hypertensive syndromes are also a cause of perinatal morbidity and mortality, mainly from intrauterine growth restriction due to utero-placental insufficiency and complications related to prematurity.6 Even mild hypertension is associated with greater risk for prematurity and newborns who are small for gestational age.7
Although much is known of the pathophysiology of PE, its etiology is still unclear. Increased blood pressure (BP) in PE can be considered a compensatory mechanism for reduced maternal–fetal blood flow.8 Several studies have suggested a possible role for the nitric oxide synthase gene and the HLA system in the genesis of PE, which would fit within a wider picture of maternal immune responses to the trophoblast that lead to defective placentation, activation of the inflammatory cascade and endothelial dysfunction.4
There is agreement that a hypertensive emergency must be treated, but the best drug to use, and the importance for the health of both mother and fetus of maintenance antihypertensive therapy and treatment of non-severe hypertension, are less clear.
Objective and methodsThe aim of this review was to summarize current knowledge on the diagnosis, pathophysiology, prediction, prevention and treatment of hypertension in pregnancy, focusing on the most recent studies on pathophysiology, prevention, identification of high-risk groups, and antihypertensive therapy in cases of severe hypertension, and analyzing the evidence on the efficacy and safety of the available drugs.
Guidelines on hypertension in pregnancy were reviewed, particularly those of the Society of Obstetricians and Gynaecologists of Canada, the British Columbia Reproductive Care Program, the National Institute for Health and Clinical Excellence (UK), and the World Health Organization. The Medline, PubMed and Cochrane Library databanks were searched for the most recent evidence, using the search terms eclampsia, pre-eclampsia, hypertension/pregnancy, pre-eclampsia/prevention, gestational hypertension, and hypertensive disorders of pregnancy. The concepts explored were diagnosis, assessment, classification, prediction (using clinical or laboratory markers), prevention, prognosis and treatment.
Diagnosis and classificationClassification of the hypertensive syndromes of pregnancy is based on the two main manifestations of PE: hypertension and proteinuria, and so rigorous measurement of BP and proteinuria is of particular importance.
According to the guidelines of the Canadian and British Hypertension Societies, BP in a pregnant woman should be measured as follows:
- •
The patient should be seated at 45° with the arm at the level of the chest;
- •
An appropriate-sized cuff should be used;
- •
A manual sphygmomanometer should be used. Automated devices tend to underestimate systolic and diastolic pressure by 5–15mmHg in pregnancy. The suitability of such devices for women with suspected or confirmed PE has only been tested in a small number of models and their use should be limited to assessment of BP variation in low-risk patients.9,10 In high-risk cases a mercury sphygmomanometer is indicated;
- •
Phase 5 Korotkoff sounds should be used to indicate diastolic BP11;
- •
Ambulatory BP monitoring in normotensive or mildly hypertensive pregnant women has not been assessed in randomized clinical trials, and its value in terms of maternal and fetal outcomes is unknown.12
Gestational hypertension is defined as systolic BP≥140mmHg and/or diastolic BP≥90mmHg on at least two occasions after the 20th week of pregnancy in a previously normotensive woman. The interval between BP measurements should be a minimum of 4–6hours and a maximum of seven days.
Diastolic BP is a better predictor of adverse pregnancy outcomes than systolic BP; a diastolic BP of 90mmHg is the level above which perinatal morbidity is increased in non-proteinuric hypertension.13 Severe hypertension is defined as systolic BP≥160mmHg or diastolic BP≥110mmHg and measurement should be repeated after 15min to confirm the diagnosis. These cutoffs were selected on the basis of evidence of a significantly increased risk of stroke in pregnant women with BP above these levels.14
The recommendations for measurement of proteinuria are as follows:
- •
All pregnant women should be assessed for proteinuria;
- •
When the suspicion of pre-eclampsia is low, a urine test strip may be used. If this gives a negative or inconclusive result, a more reliable test (24-hour urine protein/creatinine ratio) is recommended if the degree of suspicion is high.
A diagnosis of proteinuria is suggested by a score of 2+ on the test strip and confirmed by levels over 0.3g/dl in 24-hour urine or a ratio of protein (in mg) to creatinine (in mmol) of over 30 in a urine sample. However, it is important to bear in mind that target-organ damage in PE can occur in the absence of significant proteinuria. Of pregnant women who develop pre-eclampsia, 20% only present hypertension in the week before the first seizure, 10% only present proteinuria, and 10% present neither.15
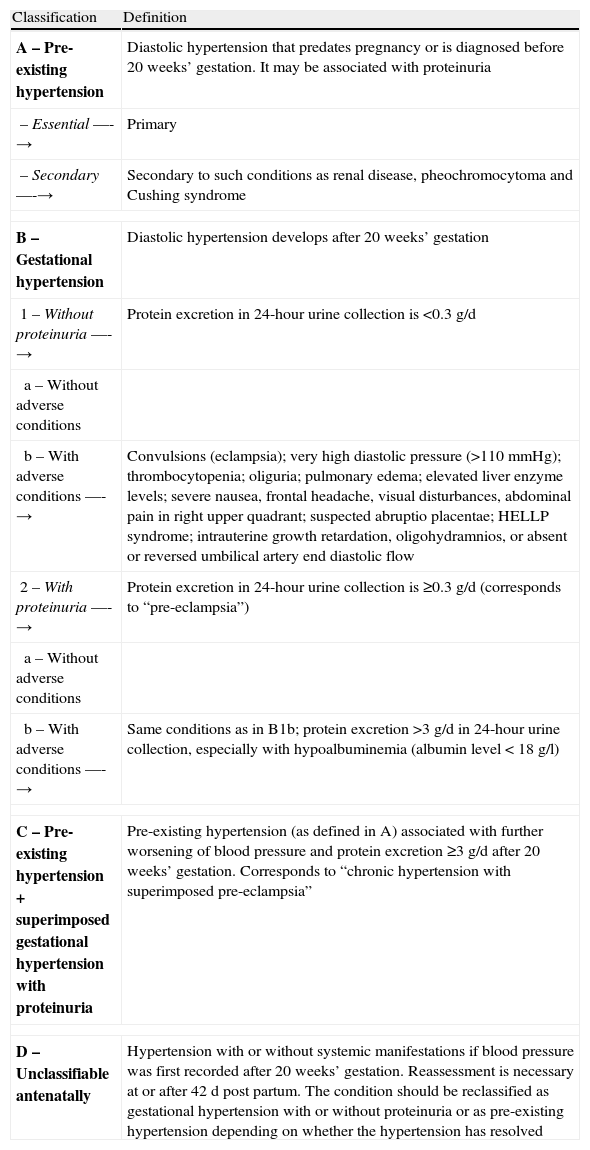
Table 1 shows the classification of hypertensive disorders of pregnancy proposed by the Canadian Hypertension Society and validated by the International Society for the Study of Hypertension in Pregnancy.
Classification of hypertensive disorders of pregnancy proposed by the Canadian Hypertension Society.
| Classification | Definition |
| A – Pre-existing hypertension | Diastolic hypertension that predates pregnancy or is diagnosed before 20 weeks’ gestation. It may be associated with proteinuria |
| – Essential —-→ | Primary |
| – Secondary —-→ | Secondary to such conditions as renal disease, pheochromocytoma and Cushing syndrome |
| B – Gestational hypertension | Diastolic hypertension develops after 20 weeks’ gestation |
| 1 – Without proteinuria —-→ | Protein excretion in 24-hour urine collection is <0.3g/d |
| a – Without adverse conditions | |
| b – With adverse conditions —-→ | Convulsions (eclampsia); very high diastolic pressure (>110mmHg); thrombocytopenia; oliguria; pulmonary edema; elevated liver enzyme levels; severe nausea, frontal headache, visual disturbances, abdominal pain in right upper quadrant; suspected abruptio placentae; HELLP syndrome; intrauterine growth retardation, oligohydramnios, or absent or reversed umbilical artery end diastolic flow |
| 2 – With proteinuria —-→ | Protein excretion in 24-hour urine collection is ≥0.3g/d (corresponds to “pre-eclampsia”) |
| a – Without adverse conditions | |
| b – With adverse conditions —-→ | Same conditions as in B1b; protein excretion >3g/d in 24-hour urine collection, especially with hypoalbuminemia (albumin level<18g/l) |
| C – Pre-existing hypertension+superimposed gestational hypertension with proteinuria | Pre-existing hypertension (as defined in A) associated with further worsening of blood pressure and protein excretion ≥3g/d after 20 weeks’ gestation. Corresponds to “chronic hypertension with superimposed pre-eclampsia” |
| D – Unclassifiable antenatally | Hypertension with or without systemic manifestations if blood pressure was first recorded after 20 weeks’ gestation. Reassessment is necessary at or after 42d post partum. The condition should be reclassified as gestational hypertension with or without proteinuria or as pre-existing hypertension depending on whether the hypertension has resolved |
A simplified classification is found in the International Classification of Diseases (ICD) (Table 2).
Hypertensive disorders in pregnancy: International Classification of Diseases (ICD) classification.
| O10 | Pre-existing essential hypertension complicating pregnancy, childbirth and the puerperium |
| O11 | Pre-existing hypertensive disorder with superimposed proteinuria |
| O12 | Gestational (pregnancy-induced) edema and proteinuria without hypertension |
| O13 | Gestational (pregnancy-induced) hypertension without significant proteinuria |
| O14 | Gestational (pregnancy-induced) hypertension with significant proteinuria |
| O14.1 | Severe pre-eclampsia |
| O15 | Eclampsia |
| O16 | Unspecified maternal hypertension |
Despite being a major cause of maternal and fetal morbidity and mortality, the mechanisms responsible for the pathogenesis of pregnancy-induced hypertension (PIH) have not yet been fully elucidated. Studies during the past decade suggest that the initiating event is reduced utero-placental perfusion as a result of abnormal invasion of spiral arterioles by the extravillous cytotrophoblast and consequent reduction of blood flow to the intervillous space. The resulting placental ischemia would lead to widespread activation/dysfunction of the maternal vascular endothelium, which results in enhanced formation of endothelin and thromboxane, increased vascular sensitivity to angiotensin II, and decreased formation of nitric oxide and prostacyclin. The oxidative stress and systemic vasospasm associated with endothelial dysfunction would lend support to the model of target-organ damage outlined.16 A study by Gilbert et al. corroborates this theory by providing evidence linking placental ischemia/hypoxia and the production of molecules such as tumor necrosis factor-α, angiotensin II type 1 receptor antibodies, interleukin-6, and a variety of antiangiogenic substances, leading to widespread dysfunction of the maternal vascular endothelium, production of vasoconstrictors such as endothelin, thromboxane and angiotensin II, and reactive oxygen species (ROS), and decreased formation of vasodilators.17 These endothelial abnormalities cause hypertension by impairing natriuresis, increasing total peripheral resistance and glomerular endotheliosis. The authors stress the need for further studies to clarify the link between placental ischemia and maternal cardiovascular alterations in order to develop effective preventive therapies.
Stennet et al. add further elements to the equation, suggesting that cytokines and ROS released by the placenta may increase vascular permeability, cross the blood–brain barrier, and affect sympathetic tone and the neuronal control mechanisms of BP.18 New data have also been furnished by Furuya et al., who suggest that the failure of trophoblasts to sufficiently invade the placental bed only partially explains PIH, and that other factors include (i) the inappropriate secretion into the maternal circulation of proinflammatory substances such as endoglin and the soluble form of vascular endothelial growth factor (VEGF) receptor-1 (an endogenous inhibitor of the angiogenic protein VEGF); (ii) direct damage to the endothelium by shear stress of uteroplacental blood flow; and (iii) microfocal fetal–placental hypoxia of unknown cause.19 The two-stage model – poor placentation in the early gestational period (stage I) and maternal systemic endothelial dysfunction in the later period (stage II) – is thus partially called into question; the authors suggest that impaired placental vasculogenesis does not in itself explain the clinical spectrum of pre-eclampsia.
A 2009 study in the Journal of Experimental Medicine assessing the association between intrauterine growth restriction in pregnant women with hypertension and angiotensin II type 1 receptor autoantibodies suggested that these autoantibodies have direct harmful effects on fetal development by inducing apoptosis of placental and trophoblast cells.20 Shah et al. corroborated these findings, adding that vascular endothelial growth factor and reduced placental growth factor (through increases in soluble tyrosine kinase-1, an antiangiogenic protein) may play a role in the development of proteinuria and other renal injury-mediated manifestations in pre-eclampsia.21
A study by Wang et al. added to the controversy by suggesting that the endothelial dysfunction seen in PE is not directly linked to fetal growth restriction associated with abnormal umbilical artery flow. Maternal plasma from pregnancies with umbilical placental vascular disease did not affect endothelial cell expression of nitric oxide synthase in vitro, which led the authors to suggest that the placental vascular pathology may be the primary event and that reduced uteroplacental circulation is secondary.22 This finding prompted further studies. Aardema et al. also raised the possibility that other factors besides defective placentation are implicated in the pathophysiology of PIH and PE. Their study sets out to test the hypothesis that PIH and PE with an early onset and poor pregnancy outcome is associated with defective placentation (inadequate spiral arteriole dilatation and subsequent reduced uteroplacental perfusion), whereas PIH and PE with normal pregnancy outcome is not. They measured the uterine artery pulsatility index (uteroplacental resistance to blood flow) by Doppler ultrasound and found that it was significantly higher in pregnancies with complicated PE but was normal in women who developed PIH/PE, but had a good pregnancy outcome. This indicates that only cases with poor outcomes are associated with defective placentation and supports the concept of heterogeneous causes of hypertensive disorders of pregnancy.23
This concept was strongly supported by Cross, who suggested that PE can be initiated by at least three independent mechanisms: pre-existing maternal hypertension that is exacerbated by pregnancy, elevated levels of angiotensin II in the maternal circulation by placental over-production of renin (placental renin-angiotensin system), and primary placental pathology, which prevents the placenta from contributing to the normal cardiovascular adaptations of pregnancy. Identification of genetic risk factors will thus only be possible when the disease has been separated into different subtypes according to the etiological mechanisms involved.24
Genetic study may have an important role in the future; mutations in the angiotensinogen gene are known to be associated with greater predisposition to PIH.19
Predicting pre-eclampsiaA good test for predicting PE should be simple, rapid, non-invasive, inexpensive and easy to apply. The results should be reliable and reproducible, with high sensitivity and specificity. Ideally, it should provide an opportunity for early preventive therapy.
The ability to predict PE within a reasonable time from symptom onset has improved somewhat in the last decade. However, these improvements have not been significant, and the search continues for the best way to predict this complication of pregnancy.
Various risk markers for PE are known, including personal or family history of PIH or PE, pre-existing hypertension, older maternal age in the first pregnancy, maternal obesity,25 and history of renal disease and/or thrombophilia (due to factor V Leiden heterozygosity, antiphospholipid syndrome, or prothrombin gene mutations).26 However, none of these risk factors has sufficient positive predictive value to be used in isolation.
Recent research has raised the possibility that reasonable risk prediction can be achieved by measuring serum levels of substances involved in the pathogenesis of the disease or by assessing uterine artery flow by Doppler ultrasound.
A study by Boulanger et al. stressed the need for rapid and reliable methods for quantifying levels of antiangiogenic proteins such as soluble tyrosine kinase-1 and endoglin produced in excess by the placenta before the clinical manifestations of PE appear.27 Baweja et al. demonstrated that measuring urinary albumin using high-performance liquid chromatography gives a significantly more reliable urinary albumin/creatinine ratio than conventional methods. According to the authors, a ratio of >35.5mg/mmol predicted pre-eclampsia well before the onset of clinical manifestations.28
Various studies on uterine artery Doppler imaging, specifically estimation of indices of flow resistance (including the uterine artery pulsatility index) and notching in the pulsed Doppler spectrum, have suggested that this may be an effective way of predicting PE.29 Cnossen et al. reported more accurate prediction by this technique when performed in the second trimester, as well as the ability to predict intrauterine growth restriction (although with less predictive value).30 Papageorghiou and Roberts confirmed these findings, adding that uterine artery Doppler screening can identify women in whom biochemical markers should be measured.31 Despite the enthusiasm with which these studies have been met, Doppler uterine artery analysis is unable in isolation to predict PE risk, as it identifies only 40–60% of those who subsequently develop PE and 20% of those who develop fetal growth restriction.32 However, when combined with assessment in the first trimester of serum markers associated with the pathophysiology of PE, particularly placental protein 13, placental growth factor, VEGF and soluble tyrosine kinase-1, it may predict up to 90% of cases of severe preeclampsia for a false positive rate of 9%.33
Despite the lack of randomized trials showing the benefit of ambulatory blood pressure monitoring (ABPM) in predicting PE, some authors recommend serial BP measurement by this method, based on various observational studies and small trials. A study published in Arquivos Brasileiros de Cardiologia concluded that certain data from ABPM can predict PIH, particularly diastolic pressure load during wakefulness, diastolic and systolic pressure load during sleep, and pressure variability and maximum diastolic pressure during sleep. Specifically a maximum diastolic arterial pressure on ABPM during sleep of ≥64mmHg presented an odds ratio of 6 for PIH with a sensitivity of 80% and a specificity of 60%.34
A few years ago the World Health Organization began a large prospective observational study to assess the value of measuring levels of two antiangiogenic substances (soluble endoglin and soluble tyrosine kinase-1) and one angiogenic substance (placental growth factor) for predicting PE. The aim is to discover whether reversing the angiogenic imbalance in PE by adding exogenous angiogenic factors can be achieved in practice, thereby correcting the syndrome.
In the absence of a test that can predict PE in isolation, multivariate models have been developed, although as yet with little success. Notable among them is the model recently developed by von Dadelszen et al. and which is currently at an advanced stage of investigation. The fullPIERS model was developed in a prospective multicenter study in women who were admitted to tertiary obstetric centers with PE or who developed PE after admission and was first published in January 2011. The model predicted adverse maternal outcomes (mortality or other serious complications of PE) occurring within the first 48hours after eligibility with a high degree of reliability and performed well up to seven days after eligibility, and brings new hope of identifying women at increased risk of adverse outcomes up to seven days before complications arise, so that aggressive preventive and therapeutic measures can be taken.35 Further details of the model are due to be released shortly.
Prevention and treatmentThere has been extensive research into the prevention of PE, although current guidelines focus mainly on preventing its complications. Non-severe PIH may have an adaptive function; neonatal morbidity is lower, and neurological development is better, in small for gestational age babies with mildly hypertensive as opposed to normotensive mothers.
Several studies have assessed the preventive value of various therapies, including inhibition of placental phosphodiesterase-5 to reverse the placental vasoconstriction that produces the ischemia/hypoxia of PE,36 while low-dose aspirin (75–100mg) is accepted as a preventive measure in high-risk women (with PIH, gestational diabetes or previous PE).37 The dose should be determined on the basis of platelet function testing.38 Some antihypertensive agents also inhibit platelet activation in primary hypertension and their benefits in PIH therefore go beyond simply reducing BP.
Calcium supplementation is beneficial in cases of low calcium intake and in pregnancies at high risk for early complications. Supplementation with antioxidants (vitamins C and E), zinc, melatonin, coenzyme Q10, omega-3 fatty acids and protein has not shown benefits and is not currently recommended.
Antihypertensive therapy does not prevent PE or its complications, although it reduces by almost half the incidence of severe hypertension in women with mild to moderate hypertension. It cannot be recommended specifically for the prevention of PE until it has been demonstrated that reducing maternal BP is not outweighed by negative fetal outcomes.
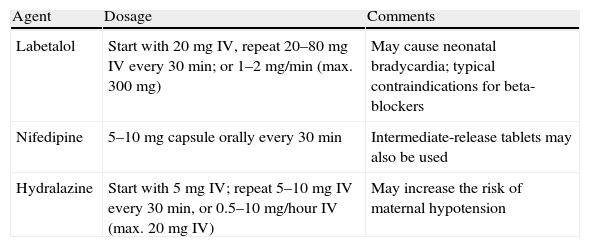
Severe hypertension (BP>160/110mmHg) should be treated in order to decrease maternal morbidity and mortality. Most women with severe hypertension in pregnancy will have PE, and most of those will have had normal BP in the recent past. Such marked BP elevations are considered urgencies. Labetalol, nifedipine and hydralazine are recommended for the treatment of severe PIH, aiming to reduce BP to <160/110mmHg. Table 3 lists the main drugs used in severe PIH and their doses. Sodium nitroprusside should only be used as a last resort if all other measures have failed to obtain BP<160/110mmHg.
Drugs recommended for treatment of severe pregnancy-induced hypertension.
| Agent | Dosage | Comments |
| Labetalol | Start with 20mg IV, repeat 20–80mg IV every 30min; or 1–2mg/min (max. 300mg) | May cause neonatal bradycardia; typical contraindications for beta-blockers |
| Nifedipine | 5–10mg capsule orally every 30min | Intermediate-release tablets may also be used |
| Hydralazine | Start with 5mg IV; repeat 5–10mg IV every 30min, or 0.5–10mg/hour IV (max. 20mg IV) | May increase the risk of maternal hypotension |
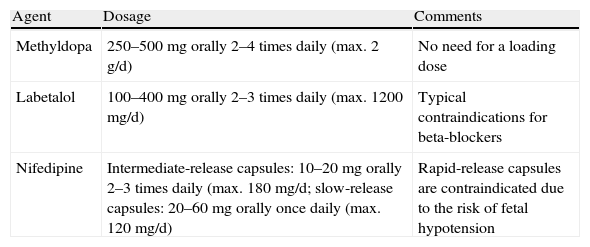
Treatment of non-severe hypertension (BP 140–159/90–109mmHg) aims to achieve values of 130–155/80–105mmHg (or 130–139/80–89mmHg in women with renal or cardiovascular comorbidities). Initial treatment should be with methyldopa, labetalol, other beta-blockers such as metoprolol, pindolol or propanolol (atenolol is not recommended since unlike the other beta-blockers mentioned, it is associated for unknown reasons with intrauterine growth retardation), or oral dihydropyridine calcium channel blockers (preferably slow- or intermediate-release nifedipine). Angiotensin receptor blockers and angiotensin-converting enzyme inhibitors are contraindicated due to their toxicity, particularly nephrotoxicity. Thiazide diuretics used after the first trimester are not associated with adverse maternal or fetal outcomes, but neither do they prevent PE or severe hypertension. Table 4 lists the main drugs used in the treatment of non-severe PIH and their doses.
Drugs recommended for treatment of non-severe pregnancy-induced hypertension (BP 140–159/90–109mmHg).
| Agent | Dosage | Comments |
| Methyldopa | 250–500mg orally 2–4 times daily (max. 2g/d) | No need for a loading dose |
| Labetalol | 100–400mg orally 2–3 times daily (max. 1200mg/d) | Typical contraindications for beta-blockers |
| Nifedipine | Intermediate-release capsules: 10–20mg orally 2–3 times daily (max. 180mg/d; slow-release capsules: 20–60mg orally once daily (max. 120mg/d) | Rapid-release capsules are contraindicated due to the risk of fetal hypotension |
Treatment of non-severe hypertension is the subject of debate because of potential harmful effects on fetal development. Two meta-analyses on this subject found a significant association between treatment-induced decreases in maternal mean arterial pressure and prematurity and small for gestational age infants,39,40 reinforcing the idea that antihypertensive therapy in itself does not reduce maternal morbidity in PE or eclampsia.
ConclusionPregnancy-induced hypertension is still a little-understood entity, despite the enormous impact of its complications on maternal and fetal outcomes. There is consensus that antihypertensive therapy is essential in cases of severe hypertension, but there is disagreement concerning non-severe cases due to the risk of adverse fetal outcomes. Recent advances in our understanding of the pathophysiology of PE have raised expectations that effective methods to predict the condition and its complications will be developed, based on specific markers of endothelial dysfunction and/or antiangiogenic proteins, the detection and quantification of which, complemented by Doppler assessment of uterine artery flow, will help with timely identification of subgroups of pregnant women at risk of developing complications. It will then be possible to apply preventive measures such as low-dose aspirin and calcium supplementation, plan the optimum timing for delivery, potentially reverse antiangiogenic status using exogenous angiogenic substances, and use antihypertensive therapy appropriately, with greater confidence and with less risk for both mother and fetus.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Barra, S. Hipertensão Arterial na Grávida: o actual estado da arte. Rev Port Cardiol 2012. doi:10.1016/j.repc.2012.04.006