The authors report the case of a patient diagnosed with both hypertrophic cardiomyopathy and aortic stenosis. Due to clinical deterioration, additional investigation was performed, and a high left ventricular outflow tract gradient was identified. Correct identification of the condition causing the symptoms was challenging, and involved several imaging techniques, the contribution of transesophageal echocardiography being crucial. The final diagnosis of severe aortic stenosis led to successful valve replacement surgery. The presence of these two conditions in the same patient has been documented, although it is uncommon. This association poses particular diagnostic and therapeutic challenges, which are discussed in this paper.
Os autores apresentam o caso de uma doente com os diagnósticos de miocardiopatia hipertrófica e estenose aórtica, na qual foi identificada a presença de um gradiente elevado ao nível do trato de saída do ventrículo esquerdo. O reconhecimento da patologia responsável pela sintomatologia foi desafiante, com envolvimento de várias técnicas de imagem, tendo sido fundamental a contribuição do ecocardiograma transesofágico. O diagnóstico final de estenose aórtica severa conduziu à referenciação para cirurgia de substituição valvular, com sucesso. A presença destas duas patologias em simultâneo num mesmo doente é conhecida, embora incomum. A sua combinação cria importantes desafios diagnósticos e terapêuticos, os quais serão objeto de discussão neste artigo.
Aortic stenosis (AS) and hypertrophic cardiomyopathy (HCM) are two conditions that can cause hemodynamic gradients in the left ventricular outflow tract (LVOT).1 In both cases the presence of significant obstruction has clinical, therapeutic and prognostic implications.1–3
The presence of both of these conditions in the same patient has been documented, although it is uncommon. This association poses particular diagnostic and therapeutic challenges.4 Meticulous echocardiographic assessment is required for correct identification of the cause of the obstruction,5 although this can be complicated, and the result can lead to different therapeutic options.6,7
This case report aims to discuss the complexity of such cases.
Case reportA 68-year-old woman, with a history of hypertension, dyslipidemia, obesity and breast cancer (treated by left mastectomy and adjuvant chemotherapy and radiotherapy in 1998), was referred for cardiology consultation in April 2011 to investigate chest pain; she had no other cardiovascular symptoms. On physical examination, auscultation revealed a grade III/VI systolic murmur audible at the right second intercostal space, crescendo-decrescendo and radiating to the carotids; the murmur became less intense with the Valsalva maneuver and on standing up, and increased with squatting.
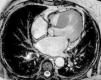
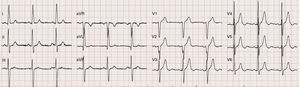
A previous electrocardiogram had shown sinus rhythm with voltage criteria for left ventricular hypertrophy without overload (Figure 1), while transthoracic echocardiography (TTE) (described as “technically very difficult”) had revealed concentric hypertrophy of the left ventricle (LV) with no wall motion abnormalities and with preserved global systolic function and a calcified aortic valve (AV) with moderate stenosis (mean left ventricle/aorta [LV/Ao] gradient of 21 mmHg).
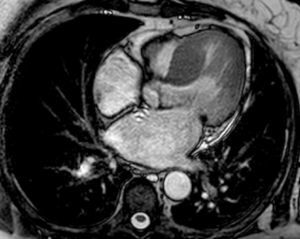
Given the patient's low pretest probability of coronary artery disease, coronary computed tomography angiography was performed, which identified mild coronary calcification (calcium score 54 Agatston units) with no endoluminal obstruction, and also revealed thickening (22 mm) of the interventricular septum (IVS). Suspicion of HCM prompted investigation by magnetic resonance imaging (MRI), which confirmed the diagnosis of asymmetric HCM with hypertrophy of the basal and mid IVS (22 mm), all other walls being of normal thickness; non-dilated LV with ejection fraction of 69% and LV mass index of 78 g/m2; moderately dilated left atrium (area 33 cm2); and no late gadolinium enhancement (Figure 2). The patient presented no risk factors for sudden cardiac death and genetic study for Fabry disease was negative; screening for classic mutations in sarcomere protein genes is in progress.
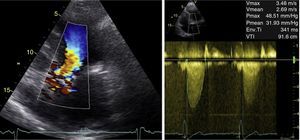
At 18-month follow-up she presented worsening functional capacity with dyspnea on moderate exertion (New York Heart Association class II). TTE was repeated and showed marked LV hypertrophy of the basal IVS and good global systolic function; an apparently tricuspid AV, calcified, with reduced opening, that could not be assessed by planimetry; and a calcified mitral valve with systolic anterior motion (SAM). Doppler study revealed accelerated flow beginning in the LVOT, with peak velocity at mid-systole and peak and mean LV/Ao gradient of 49 mmHg and 32 mmHg, respectively, supporting the hypothesis of a fixed obstruction (Figure 3). No late-systolic velocity peak was observed, with or without the Valsalva maneuver.
Transthoracic echocardiography: (left) color Doppler showing turbulent flow beginning in the left ventricular outflow tract; (right) continuous-wave Doppler showing rounded waveform with mid-systolic peak (bell-shaped) and peak and mean left ventricular/aortic gradient of 49 mmHg and 32 mmHg, respectively.
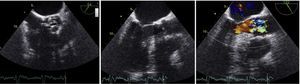
In view of the limitations of TTE, transesophageal echocardiography (TEE) was performed, which revealed a malformed AV with marked calcification and fusion of the noncoronary and left coronary leaflets, with an area estimated by planimetry of 0.6 cm2 (0.27 cm2/m2) (Figure 4). Color Doppler study clearly differentiated laminar flow in the LVOT and turbulent flow through the AV throughout systole, confirming the suspicion of obstruction of the valve only (Figure 4).
Transesophageal echocardiography: (left) malformed aortic valve with area calculated by planimetry of 0.6 cm2; (center) left ventricular outflow tract without visible obstruction; (right) color Doppler differentiating laminar flow in the left ventricular outflow tract and turbulent flow through the aortic valve, confirming obstruction at the level of the valve.
Invasive hemodynamic study showed a peak-to-peak LV/Ao gradient of 52 mmHg and no intraventricular gradient, and excluded significant coronary artery disease.
A 22-mm Medtronic Hall mechanical valve was implanted surgically in aortic position. At six-month follow-up the patient presented improved functional capacity and TTE revealed a normally functioning aortic valve.
DiscussionThis case report highlights the difficulties of investigating a patient with both HCM and AS, particularly in assessing the severity of each condition and determining which is functionally more important. Identifying the cause of the high LVOT gradient as AS led to the patient being referred for valve replacement surgery, which resolved the obstruction and improved symptoms.
Assessment of such patients is based on a thorough echocardiographic assessment of the LVOT region.4,5 Color and pulse wave Doppler study are essential to locate the level at which flow acceleration occurs,8 but TTE does not always provide definitive information.
Continuous-wave Doppler can quantify the obstruction, and the shape of the velocity waveform is particularly useful in differentiating fixed and dynamic obstruction.9,10 Obstructive HCM is characterized by an LVOT or, less commonly, a midventricular gradient11 that changes with variations in preload, afterload and contractility.12 Since it is predominantly dynamic, the gradient develops at end-systole and the waveform is dagger-shaped. By contrast, AS results in a fixed obstruction to LV outflow throughout systole with peak velocity at mid-systole, giving a bell-shaped waveform.5,9,10
In the case presented, continuous-wave Doppler study indicated the presence of a fixed obstruction; however, the existence of SAM and marked septal hypertrophy raised the suspicion of a dynamic subaortic obstruction. When assessing these patients, particular care should be taken in interpreting the Doppler waveform, since the two patterns may overlap and the presence of a second gradient may be overlooked.4 If the level of suspicion is high, and TTE study is inconclusive, TEE should be used.4,13
TEE also plays an important role in screening for other conditions that can cause a fixed LVOT obstruction, such as HCM itself (due to fibrous tissue formation caused by contact between the mitral valve and the IVS), accessory mitral tissue, subaortic ridge, and tunnel subaortic stenosis.12 In the present case, TEE was crucial in identifying flow acceleration at the valve and in excluding other conditions.
The use of cardiac MRI to measure LVOT velocities has been described, but Doppler TTE has been more thoroughly validated.13 In some cases an accurate hemodynamic study can only be obtained by invasive means.14
Assessment of the severity of AS in patients with suspected obstructive HCM poses particular challenges and there is little information available on the subject.5,9,14 Use of the modified Bernoulli equation (ΔP=4v2) is based on certain assumptions that mean it cannot be used in patients with serial stenoses.14 When flow velocity exceeds 1.0 m/s, the peak gradient can be estimated using the formula 4(v2max−v2 proximal), but calculating the mean gradient is more complex and is not easy to apply in clinical practice.9 Accurate measurement of gradients may only be possible by means of an invasive hemodynamic study.14
The continuity equation for measuring valve area cannot be used in the presence of LVOT obstruction.9 Planimetry is the recommended method, ideally by TEE.13 In our patient, the presence of valve malformation with marked calcification and an anatomical area of 0.6 cm2 led to a diagnosis of severe AS.
Diagnosis of HCM in a patient with significant AS is also not straightforward. It is based on the presence of LV hypertrophy, frequently asymmetric and involving the IVS, in the absence of other causes.8,11,13,15 AS is usually associated with a uniform or symmetric (i.e. concentric) distribution of LV hypertrophy,16 although an asymmetric septal distribution is reported in around 10% of cases.4,16,17 This makes diagnosis more difficult: is the hypertrophy an adaptive response to AS or is it due to concomitant HCM? In the case described here, the presence of marked septal hypertrophy and SAM favored a diagnosis of concomitant HCM. Genetic study and assessment of the evolution of ventricular hypertrophy following valve surgery may support the diagnosis. Some authors have suggested other characteristics that corroborate a diagnosis of HCM, including mitral valve abnormalities (such as lengthening of the anterior leaflet), hypertrophied or bifid papillary muscles or anteroapical displacement, and family history.4,8,14 MRI can have an important role in assessing some of these characteristics.14
ConclusionsThis paper highlights the complexity of assessing patients with HCM and severe symptomatic AS with high LVOT gradients. Echocardiographic study is a challenge, but thorough assessment is important due to its direct effect on choice of therapeutic strategy and thus on prognosis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Almeida I, Caetano F, Trigo J, et al. Gradiente elevado no trato de saída do ventrículo esquerdo: estenose aórtica, miocardiopatia hipertrófica obstrutiva ou ambas? Rev Port Cardiol. 2015;34:357.