Chagas disease (CD) is an infection caused by the protozoan flagellate Trypanosoma cruzi, and transmitted by insects of the genera Triatoma, Rhodnius and Panstrongylus. The heart is affected to varying degrees by inflammatory and destructive lesions in atrial and ventricular myocardial fibers. Several studies have demonstrated the benefits of exercise in patients with congestive heart failure (CHF), including reduced sympathetic tone and increased parasympathetic tone, the result of reduced epinephrine and norepinephrine levels, both at rest and during exercise, including at submaximal levels. It has been hypothesized that the increase in sympathetic arousal during exercise improves peripheral muscle metabolism.
ObjectivesThe objectives of this study were to select patients with chronic Chagas cardiomyopathy (CCC) with dysautonomia on 24-h Holter monitoring, assess autonomic function after rehabilitation, and determine whether it resulted in reduced daytime levels of SDNN and increased daytime and nighttime levels of pNN50 and rMSSD.
MethodsWe analyzed time-domain indices of heart rate variability through 24-h Holter monitoring before and after a supervised exercise program. We studied 18 CCC patients (five men), mean age 57.33±9.73 years, followed at the CD outpatient clinic of the National Institute of Cardiology and IPEC/Fiocruz in Rio de Janeiro, Brazil, between April 2009 and November 2010. The following tests were used to assess the severity of CCC: clinical examination, functional evaluation by cardiopulmonary stress testing, electrocardiogram and conventional Doppler echocardiography. The exams were performed within a month of the start and end of the exercise program, which consisted of 60-min sessions of aerobic exercise on a treadmill and resistance training three times a week for six months. The goal was to reach the patients’ heart rate target zone during training, and their rating of perceived exertion was assessed by the modified Borg scale.
ResultsThere were no statistically significant differences (p>0.05) in SDNN, pNN50 and rMSSD, probably due to the large standard deviation observed, patients’ poor adherence to the program and their low socioeconomic status, resulting in a small sample, and the short duration of the program.
ConclusionHeart rate variability parameters in patients with CCC did not undergo statistically significant changes after a six-month cardiac rehabilitation program.
A doença de Chagas (DC) é uma infeção causada pelo flagelado Trypanosoma cruzi, transmitida por insetos do género Triatoma.
Estudos têm demonstrado o resultado benéfico da prática de exercícios em portadores de insuficiência cardíaca (IC), o seu efeito de redução do tónus simpático e o aumento do tónus parassimpático relacionado com a redução dos níveis de epinefrina e noraepinefrina. Este estudo teve como objetivos: detetar pacientes com cardiopatia chagásica crónica (CCC) com disautonomia através do Holter 24h, a função autonómica após reabilitação, a redução dos níveis de SDNN/d e um aumento dos níveis de pNN50/d e pNN50/noite, bem como de rMSSD/d e noite. Foi feita uma intervenção tipo antes e depois com avaliação dos índices de domínios do tempo da variabilidade da frequência cardíaca (VFC) antes e ao final do programa de exercícios. Foram estudados 18 pacientes, sendo cinco homens, com CCC e idade média de 57,33±9,73 anos, no ambulatório de DC do Instituto Nacional de Cardiologia (INC) entre abril de 2009 e novembro de 2010. Os exercícios foram: atividade aeróbica em esteira rolante e exercícios contrarresistidos com duração de 60min e frequência de três vezes por semana. Os valores de SDNN, pNN50 e rMSSD avaliados mostram-se quase inalterados sem significância estatística (p>0,05). Concluímos que os valores dos parâmetros de VFC em portadores de CCC não se alteraram antes e após a intervenção com um programa de reabilitação cardíaca de seis meses.
Chagas disease (CD) is an infection caused by the kinetoplastid protozoan flagellate Trypanosoma cruzi, and is transmitted by insects of the genera Triatoma, Rhodnius and Panstrongylus. The most common form of transmission is by vectors, but transfusion with infected blood and transmission from mother to fetus are also of epidemiological importance.1
In the 1980s, the disease affected 18–20 million individuals in endemic areas in Latin America. World Health Organization (WHO) data published after a meeting of specialists in Argentina in 2002 indicated that 16–18 million were infected with T. cruzi. However, a 2007 study by Dias estimated the figure at 12–14 million in Latin America, with some infected individuals in Europe and North America.2
The acute phase is generally asymptomatic, with an incubation period of a week to a month after the insect bite. A large erythematous swelling (chagoma) can develop at the bite site. Other possible symptoms include fever, swollen lymph glands, appetite loss, hepatosplenomegaly, myocarditis and, more rarely, meningoencephalitis; as the trypanosomes disappear from the bloodstream, peripheral blood testing is negative for the organisms, but anti-Trypanosoma antibodies (IgG) are found in the bloodstream, an indication that CD is moving to the chronic phase.3
Patients with chronic Chagas cardiomyopathy (CCC) can remain asymptomatic for 10–20 years, but during this time the parasite continues to reproduce at a low rate, causing irreversible damage to organs including the nervous system and the heart. The consequences are apparent after one or two decades, with gradual onset of dementia, heart disease or digestive tract dilatation, the result of nerve destruction in these organs’ muscle cells. The disease is often fatal at this stage, even with treatment, usually due to heart failure.
Reduced heart rate variability (HRV) is a strong risk marker for adverse events in normal individuals and in patients with a wide range of diseases, reflecting the vital role of the autonomic nervous system (ANS) in maintaining health. Reduced HRV is found in hypertension,4–6 myocardial infarction, coronary disease7 and atherosclerosis.8 Regular exercise has been shown to increase vagal tone as a result of the physiological adaptations that occur in response to increased cardiac work due to reduced sensitivity of beta receptors. Increased parasympathetic modulation induces electrical stability in the heart, while enhanced sympathetic activity increases the heart's vulnerability and the risk of cardiovascular events.
ObjectivesThe objectives of this study were to assess changes in HRV in CD patients after a six-month exercise program and to determine whether the program resulted in reduced SDNN (daytime standard deviation of RR intervals) and increased pNN50 (daytime and nighttime percentage of differences between adjacent RR intervals >50 ms) and rMSSD (the square root of the mean squared differences of successive RR intervals).
MethodsWe selected 50 outpatients followed in the CD clinic of the National Institute of Cardiology (INC) and the Evandro Chagas Research Institute (IPEC), who treat patients with positive serology for CD by at least two methods.
All patients were informed of the research procedures and gave their written consent. The study was approved by the Research Ethics Committee of INC and IPEC.
Patients of both sexes aged between 21 and 75 years, positive serology for CD by ELISA and indirect hemagglutination testing, not being treated with benznidazole, and socioeconomic status allowing travel to the INC three times a week for six months, were included.
Patients with pacemakers, those under treatment with stem cells, or with comorbidities preventing participation in the exercise program, including severe hypertension (≥180mmHg systolic and ≥110mmHg diastolic blood pressure), decompensated diabetes, chronic heart failure in NYHA functional class IV, chronic renal failure (serum creatinine >2.0mg/dl), anemia (hemoglobin <10g/100ml or hematocrit <30%), platelet count <120000/mm3, as well as those who were unable to give informed consent or undergo 24-h Holter monitoring and those with joint disease preventing treadmill or cycle ergometer exercise, were excluded.
The study was performed in accordance with the Declaration of Helsinki and with CONEP Resolution 196/96.
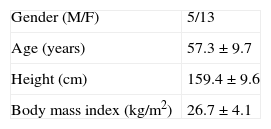
The characteristics of the study population are shown in Table 1.
Patient selectionPatients with positive serology for CCC by indirect hemagglutination testing and ELISA attending INC's outpatient CD clinic were referred to the cardiac rehabilitation unit consecutively and in accordance with the inclusion and exclusion criteria based on their medical history and thorough physical examination.
Diagnostic examsLaboratory testsThese included hemogram and blood glucose, creatinine, serum uric acid, total cholesterol, HDL cholesterol, triglycerides, serum potassium, total proteins and protein fractions, BNP, stool analysis for parasites (mainly to exclude schistosomiasis mansoni) and urine analysis.
Chest X-rayResting electrocardiogramThe electrocardiograms (ECGs) were analyzed according to the Minnesota code,9 and classified as normal, borderline or abnormal.
Cardiopulmonary stress testingTwo-dimensional echocardiography with intracavitary DopplerA conventional technique was used on the equipment available in the Echocardiography Department, with particular attention paid to cross-sections of the longitudinal, subcostal and apical short-axis views of the left ventricle when visualizing the various myocardial segments in order to analyze segmental wall motion.
Holter 24-h ambulatory electrocardiographic monitoringThe period of Holter monitoring ranged between 23h 05min and 25h 45min (mean 24h 15min), with patients maintaining their usual activities. A CardioFlash Plus (Cardios) digital system was used, with simultaneous recording of three leads. Recordings were analyzed using CardioManager 5.0 (Cardios) for processing and printing data, which were interpreted by the Arrhythmia Department of INC.
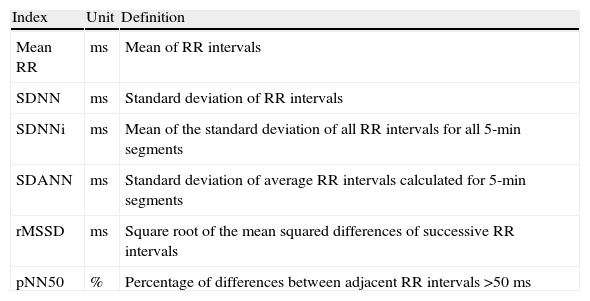
HRV was analyzed based on the time-domain indices shown in Table 2, considering the time between 1 pm and 5 pm as the daytime period of predominantly sympathetic activity, and that between 2 am and 6 am as the nighttime period of predominantly parasympathetic activity.
Definition of time-domain indices of heart rate variability.
| Index | Unit | Definition |
| Mean RR | ms | Mean of RR intervals |
| SDNN | ms | Standard deviation of RR intervals |
| SDNNi | ms | Mean of the standard deviation of all RR intervals for all 5-min segments |
| SDANN | ms | Standard deviation of average RR intervals calculated for 5-min segments |
| rMSSD | ms | Square root of the mean squared differences of successive RR intervals |
| pNN50 | % | Percentage of differences between adjacent RR intervals >50ms |
One-hour exercise sessions were held three times a week, consisting of:
- (1)
Thirty min of aerobic exercise on an Inbrasport 2000® treadmill, divided into three phases: a 5-min warm-up period with gradually increasing speed; 20min of exercise aiming to reach the patient's training heart rate target zone, taking account of their rating of perceived exertion on the modified Borg scale, in order to maintain moderate to moderate/vigorous effort; and a 5-min slow-down period to a complete stop.
- (2)
Twenty min of resistance training of the main muscle groups, consisting of two series of 10 repetitions for each of the muscle groups, programmed empirically to achieve 70% of one maximum repetition following a maximum load test to determine what each individual could achieve on one repetition of a particular exercise.
- (3)
Ten min of flexibility exercises of the main muscle groups.
The training heart rate target zone maintains the intensity of aerobic exercise within safe limits, and is determined by cardiopulmonary exercise testing; it is 5–15% above the anaerobic threshold, also known as the lactate threshold or ventilatory threshold, and 10% below the respiratory compensation point.
Statistical analysisThis was a before-and-after observational study. The data were presented as means±standard deviation or frequencies and analyzed using a mixed-effects model including a fixed effect per phase and period and a random effect for phase, period and patient. The statistical analysis was performed using R software version 2.12.1 (R Development Core Team, Vienna, Austria). Mixed-effects ANOVA was used for analysis of inference. A difference of five was considered significant, with a standard deviation of 13.8 and thus 23 patients were required to ensure 95% confidence intervals and 60% statistical power.
ResultsInitially, 50 patients were invited to undergo 24-h Holter monitoring. Of these, 25 did not attend for the exam, even after several reminders, and were thus excluded, as were a further five who had pacemakers. The remaining 20 underwent the exam; of these, two were excluded due to joint disease that prevented them exercising on a treadmill or cycle ergometer. Thus, 18 patients were selected to participate in the study, 32 being excluded according to the criteria described in the Methods section. The patients were classified in NYHA functional classes II and III.
Six patients (33.3%) were black, and 12 (66.7%) were of other ethnicities; mean age was 57.33±9.73 years. Table 1 shows the characteristics of the study population.
ECGs and two-dimensional color Doppler echocardiography were performed in 18 patients, but three (16.6%) were lost to the study.
Three patients (20%) had normal ECG and eight (53.3%) showed right bundle branch block, which was the most common ECG alteration in our population. Other alterations included non-specific changes in ventricular repolarization, left anterior hemiblock, sinus bradycardia and supraventricular extrasystoles.
On echocardiography, 13 patients (86.7%) presented ejection fraction (EF) of over 50% by Simpson's method and only two (13.3%) had EF of less than 50%.
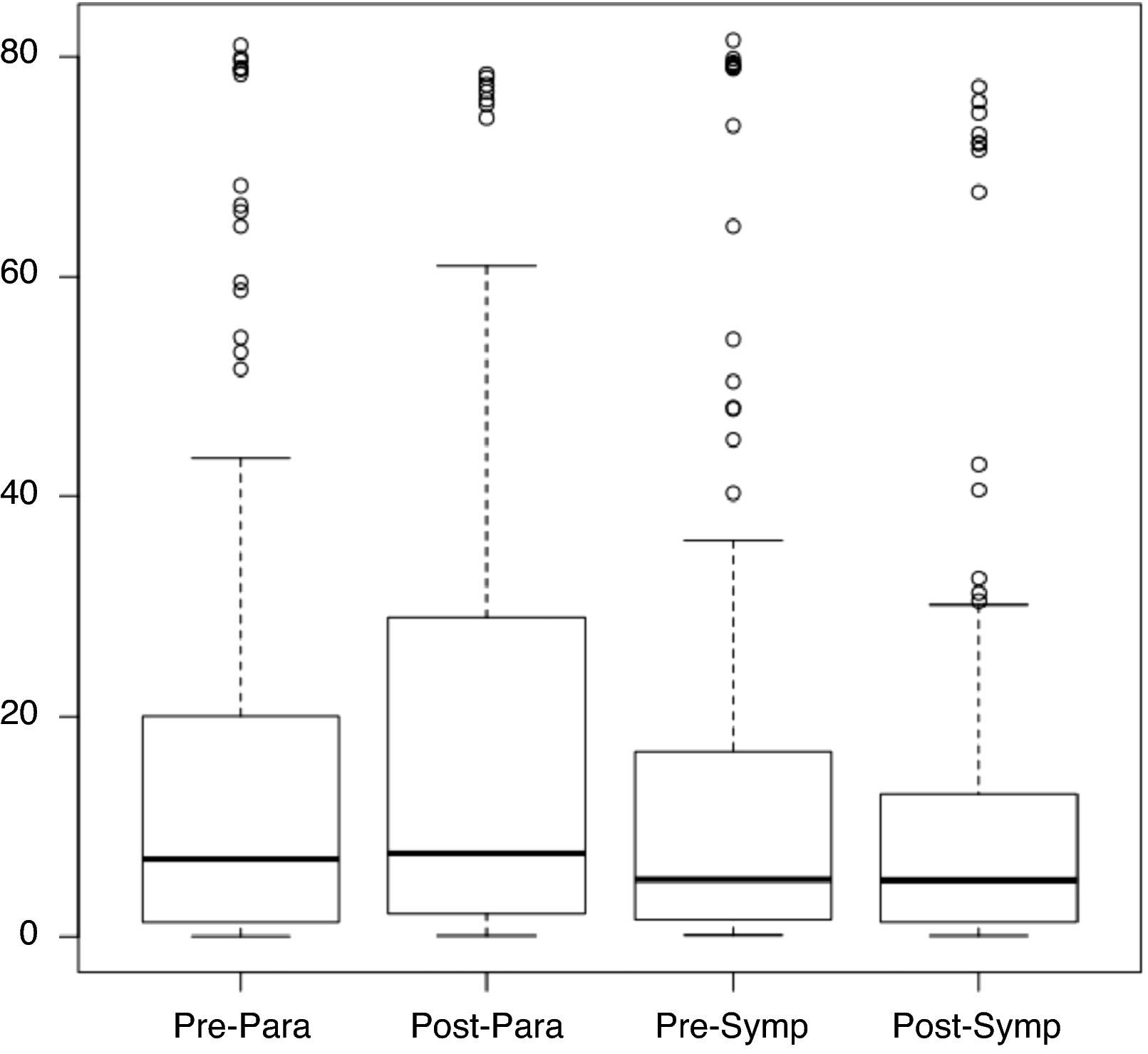
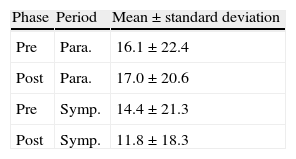
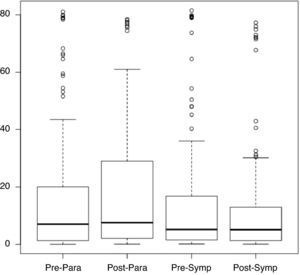
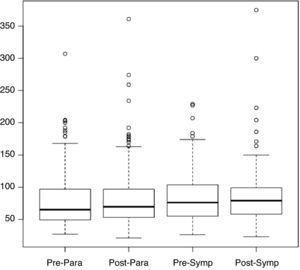
Considering the ANS as divided into sympathetic and parasympathetic components in which adrenergic and cholinergic activity are observed, respectively (corresponding to two periods: sympathetic from 1 pm to 5 pm and parasympathetic from 2 am to 6 am), Figure 1 shows the distribution of pNN50 values for the pre- and post-rehabilitation phases for each period. It can be seen that the median did not change significantly between phases, irrespective of the time of day.
Table 3 compares pNN50 values in the pre- and post-rehabilitation phases for the two periods, from which it can be seen that mean values of pNN50 remained virtually unchanged, without statistical significance (p>0.05).
Mean pre- and post-rehabilitation pNN50 values and standard deviation for the daytime and nighttime periods.
| Phase | Period | Mean±standard deviation |
| Pre | Para. | 16.1±22.4 |
| Post | Para. | 17.0±20.6 |
| Pre | Symp. | 14.4±21.3 |
| Post | Symp. | 11.8±18.3 |
Para.: parasympathetic; Post: post-rehabilitation; Pre: pre-rehabilitation; Symp.: sympathetic.
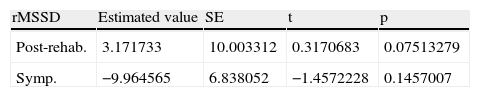
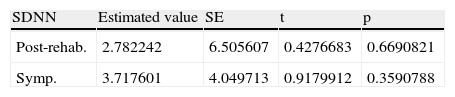
Table 4 shows the mean and standard error of rMSSD values post-rehabilitation and in the period of sympathetic activity.
rMSSD values post-rehabilitation and in the period of sympathetic activity.
| rMSSD | Estimated value | SE | t | p |
| Post-rehab. | 3.171733 | 10.003312 | 0.3170683 | 0.07513279 |
| Symp. | −9.964565 | 6.838052 | −1.4572228 | 0.1457007 |
Post-rehab: post-rehabilitation; SE: standard error; Symp.: period of sympathetic activity.
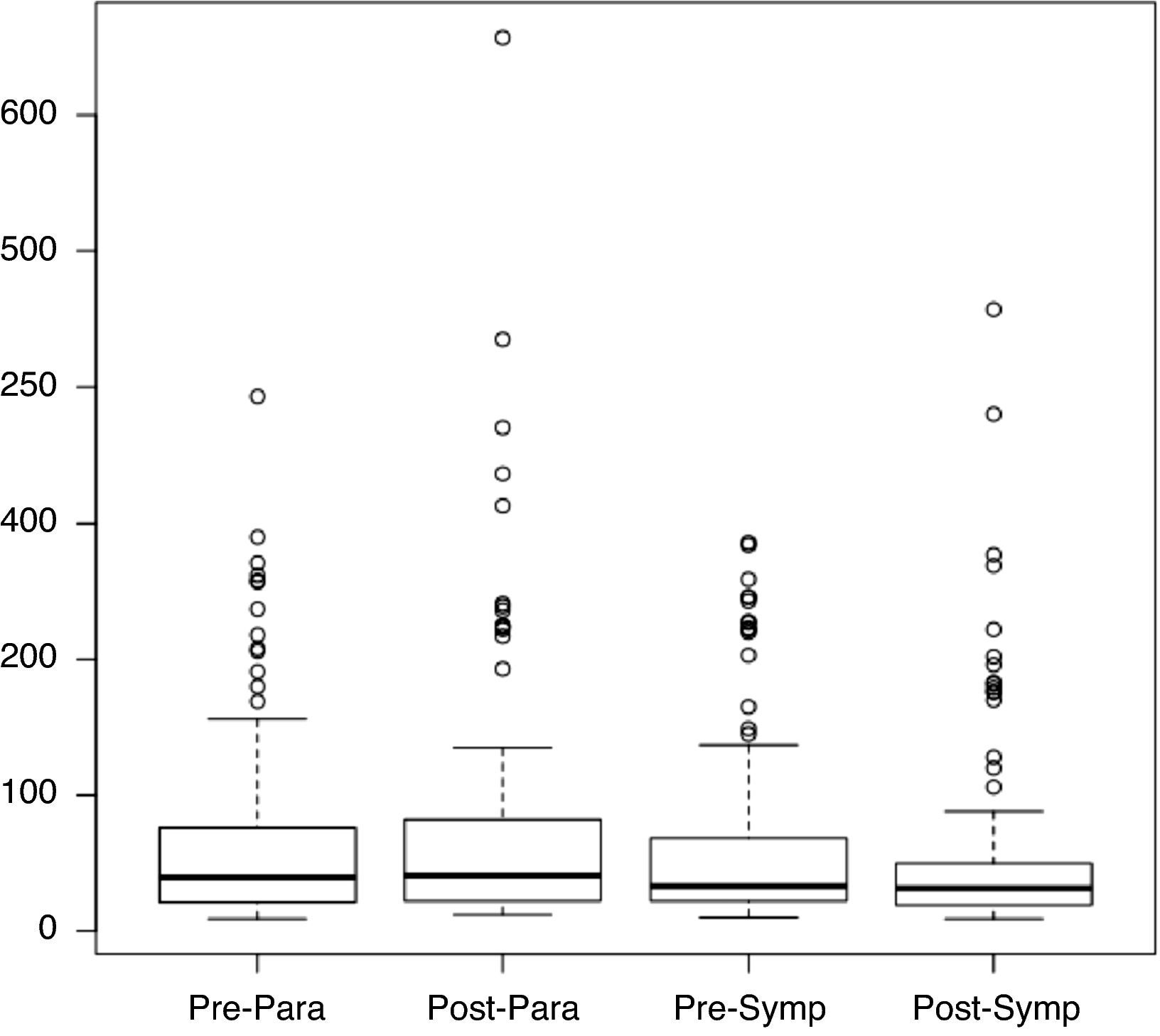
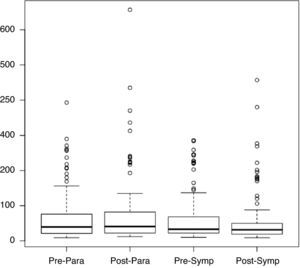
Figure 2 shows the distribution of rMSSD values in the pre- and post-rehabilitation phases, divided into the sympathetic and parasympathetic periods, from which it can be seen that the median did not change significantly between phases, irrespective of the time of day.
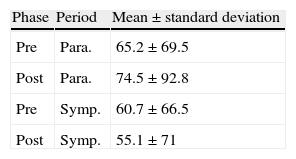
Table 5 shows rMSSD values and standard deviation for the pre- and post-rehabilitation phases and the two periods, from which it can be seen that mean rMSSD values remained virtually unchanged, without statistical significance (p>0.05).
rMSSD values and standard deviation for the pre- and post-rehabilitation phases and the two periods.
| Phase | Period | Mean±standard deviation |
| Pre | Para. | 65.2±69.5 |
| Post | Para. | 74.5±92.8 |
| Pre | Symp. | 60.7±66.5 |
| Post | Symp. | 55.1±71 |
Para.: parasympathetic; Post: post-rehabilitation; Pre: pre-rehabilitation; Symp.: sympathetic.
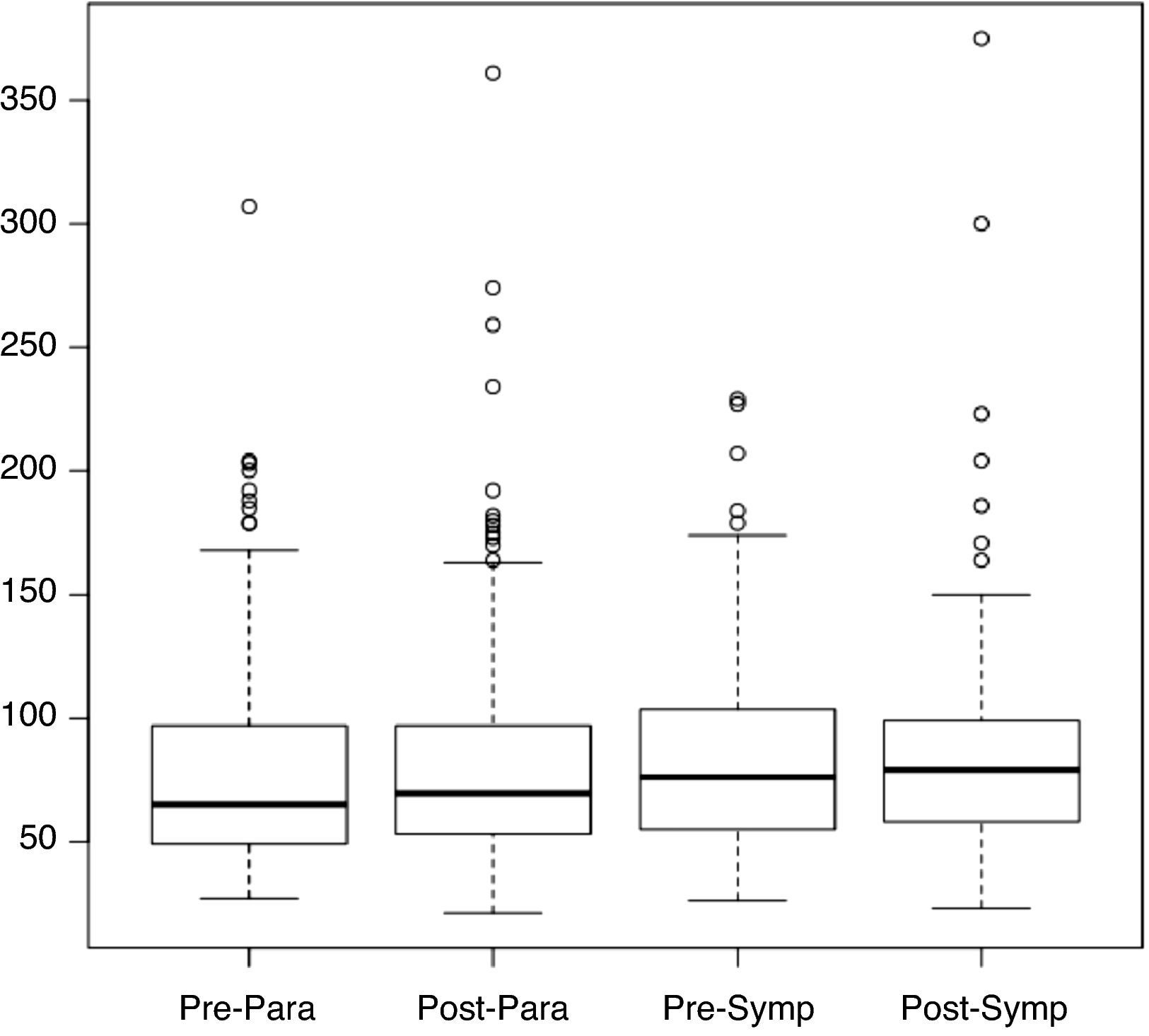
Table 6 shows SDNN values post-rehabilitation and in the period of sympathetic activity.
Figure 3 shows the distribution of SDNN values in the pre- and post-rehabilitation phases, divided into the sympathetic and parasympathetic periods, from which it can be seen that the median did not change significantly between phases, irrespective of the time of day.
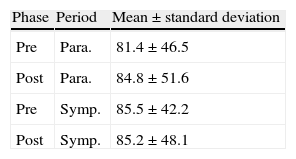
Table 7 shows SDNN values and standard deviation for the pre- and post-rehabilitation phases and the two periods.
SDNN values and standard deviation for the pre- and post-rehabilitation phases and the two periods.
| Phase | Period | Mean±standard deviation |
| Pre | Para. | 81.4±46.5 |
| Post | Para. | 84.8±51.6 |
| Pre | Symp. | 85.5±42.2 |
| Post | Symp. | 85.2±48.1 |
Para.: parasympathetic; Post: post-rehabilitation; Pre: pre-rehabilitation; Symp.: sympathetic.
Chagas disease is responsible for a large number of sudden deaths in Latin America, and is a classic example of intrinsic cardiac denervation. It can thus be considered a true experimental model to assess autonomic regulation in humans.
Cardiac rehabilitationStudies on physical activity in patients with cardiomyopathy have shown the benefits of regular exercise, such as the 10–30% increase in peak exercise capacity documented by Keteyian et al.10 in patients with chronic heart failure (CHF); Belardinelli et al.11 showed improvement in quality of life and clinical outcome, while Hornig et al.12 reported improvement in endothelial function in CHF patients; Hambrecht et al.13 showed benefits in plasma catecholamine levels, and Piepoli et al.14 reported reduced morbidity and rehospitalization. All these studies show reductions in the causes of mortality and improvements in cardiac function at rest.
Cardiac rehabilitation in chronic Chagas cardiomyopathyThe aim of this study was to assess changes in HRV in patients with CCC after a six-month cardiac rehabilitation program, which can complement medical therapy.
The mean age of our study population was 57.3 years, with a predominance of women. The sample thus included older individuals with a higher risk profile; the predominance of women did not effect interpretation of the results.
Sousa et al.15 studied the association between the HRV parameters SDNN, rMSSD and pNN50 and level of physical activity in CD patients and controls. This association was also studied in healthy individuals and CD patients using the International Physical Activity Questionnaire, which assesses levels of physical activity independently of context, and includes employment, leisure and everyday activities. The results showed that levels of normal physical activity correlated significantly with HRV indices in healthy individuals, but not in CD patients, suggesting that these patients’ dysautonomia affects this relationship. A similar result was found in our study, with no significant changes in time-domain indices of HRV during the exercise program.
Oliveira and Pedrosa16 analyzed ventilatory response during exercise among CCC patients using the Los Andes clinical and hemodynamic classification. The results indicated that the progressive deterioration in ventilatory response of these patients is more evident when peak functional capacity (VO2) is reduced than when changes are related to the Los Andes classification. Montes de Oca et al.17 assessed peripheral muscle metabolic and structural characteristics in patients with advanced CD and analyzed whether they correlated with exercise performance; they showed that such patients have decreased oxidative capacity and a shift to anaerobic metabolism in skeletal muscles. They also suggest that muscle abnormalities are related to oxygen delivery, which is probably reduced in part by the abnormal muscle microvasculature. Oliveira et al.18 compared gas exchange at rest and during exercise in CCC patients and healthy individuals grouped according to the Los Andes classification. The results showed that the functional capacity of patients in the initial phase of CCC (onset of cardiac involvement) is higher than that of patients in an advanced phase and shows a decrease that follows the loss in cardiac-hemodynamic performance. Mady et al.19 set out to characterize the functional capacity of asymptomatic CD patients with normal left ventricular function, and found that while the patients had normal left ventricular systolic function at rest, their functional capacity was significantly impaired during exercise.
In a prospective randomized trial, Lima et al.20 demonstrated that CD patients who underwent 12 weeks of exercise training showed improved functional capacity and quality of life compared to CD patients in the inactive control group. Although the study did not measure peak oxygen uptake directly, included only a small number of patients and had a short follow-up, it showed increases in exercise tolerance, distance covered on the 6-min walk test and estimated oxygen uptake, as well as improvements in quality of life; it thus represents an advance in research on the management of the cardiomyopathy associated with this important and neglected disease. Lima et al.20 demonstrated that exercise training is not only effective but feasible and is not associated with significant adverse events. Even in the context of the lack of effect on mortality and improvements in quality of life in the HF-ACTION study, Lima et al.’s study is relevant, bearing in mind the specific mechanisms involved in the pathophysiology of CCC.
Our study assessed time-domain indices of HRV through 24-h Holter monitoring before and after a supervised exercise program. Statistical analysis showed that SDNN, pNN50 and rMSSD values did not change significantly (p>0.05), probably due to the large standard deviation observed, poor patient adherence to the program, resulting in a small sample, and the short duration of the program.
Heart rate variabilityIt is assumed that neuronal uptake of norepinephrine is impaired in hypertrophic cardiomyopathy due to reduced density of beta receptors, as shown by Ianni and Mady.21
Assessment of HRV has been used to diagnose not only physiological,22 but also psychological disorders. A group of researchers analyzed whether the relationships between self-ratings of anxiety or emotional stress and parasympathetic nervous system components of HRV are independent of personality and cardiorespiratory fitness.8 They found an inverse relationship between perceived emotional stress during a week of exercise training and normalization of the high-frequency (HF) component of HRV (p=0.038), which was independent of age, gender, trait anxiety and cardiorespiratory fitness; they concluded that vagal modulation of heart period appears to be sensitive to the recent experience of persistent emotional stress, regardless of a person's level of physical fitness and disposition toward experiencing anxiety. HRV is normally used in sports medicine to assess cardiac autonomic adaptations to resistance training23 and exercise.24
Differences in HRV between fit and unfit individuals have been thoroughly investigated. Both time-domain and frequency-domain variables are higher in fit than in sedentary individuals, indicating that the former have higher HRV.
Ribeiro and Rocha25 assessed rMSSD and very low frequency, low-frequency (LF) and HF indices and found no significant differences at rest between healthy sedentary men and active patients with hypertension and myocardial infarction, suggesting that physical activity influenced autonomic modulation in these patients. Such an effect was also reported by Kropf et al.,26 who analyzed rMSSD at rest in active patients with coronary artery disease and healthy individuals and found no significant differences between the groups. Furthermore, these indices can be used to determine the influence on autonomic regulation of factors such as age,27 gender,28 and exercise.29
Heart rate variability and Chagas diseaseSartori et al.30 assessed time- and frequency-domain indices in 81 CCC patients (47 in the indeterminate phase, of whom eight were male, mean age 55.07±10.75 years, and 34 in the chronic phase, of whom eight were male, mean age 57.46±11.59 years) and compared them with a control group of 24 individuals (seven male, mean age 48.50±13.93 years). The variables SDNN, SDNNi and SDANN, rMSSD, pNN50, and LF and HF were measured in all individuals through 24-h Holter monitoring and compared by ANOVA. There were no statistically significant differences between the groups for SDNN, SDNNi or HF (p=0.05), but significant differences were found for SDANN and LF (p=0.01) between controls and both groups of CD patients; patients with CD presented statistically higher values for rMSSD and pNN50 than controls. Spectral analysis showed a reduction in sympathetic response and global impairment of autonomic function as reflected in lower SDANN values in both types of CD patients.
de Resende et al.28 assessed cardiac autonomic function in 84 individuals living in an endemic area through computerized analysis of HRV; the study population consisted of 28 elderly patients with indeterminate CD, 28 elderly individuals without the disease and 28 young adults. There were no statistically significant differences between the groups in terms of systolic and diastolic diameters or left ventricular systolic function, nor in mean RR interval. There was a statistically significant difference in variance, standard deviation, variation coefficient and pNN50 between the young and the elderly, but not between the two elderly groups. The authors concluded that the elderly groups with and without CD did not differ in terms of baseline time-domain indices of cardiac autonomic function.
Another important aspect of CD is intrinsic denervation of the ANS, as noted in the first studies on CD in Brazil. Köberle,31 a pioneer in the study of the ANS in CD, adopted the technique of neuron counts in the 1950s, and showed a reduction in the number of parasympathetic nerve cells; he thus considered that Chagas cardiomyopathy involved reduced parasympathetic activity with predominance of the sympathetic.
In autopsy studies, Köberle and colleages32,33 and Mott and Hagstrom34 demonstrated lesions of the cardiac ANS in CD patients and observed that the nerve structures of the heart could be affected in a diffuse or focal manner.
In an experimental study, Tafuri and Raso35 demonstrated the damage caused by CD to the sympathetic and parasympathetic nervous system; ganglia lesions could involve all or part of the ganglion, affecting intra- and extra-ganglion nerve fibers.
In pharmacological and hemodynamic studies, Amorim et al.36 also suggested that the sympathetic nervous system was impaired in CCC patients, making it clear that further studies are required to assess sympathetic neurons in these patients. In this respect, other diseases that affect the ANS, such as familial amyloid polyneuropathy (FAP), may alter its classic manifestations when patients exercise. FAP is a systemic amyloidosis of autosomal dominant inheritance characterized by ascending neuropathy of motor and sensory fibers and dysautonomia. The condition is associated with reduced ANS nerve fiber density, as reported by Sousa and Saraiva37 These observations mean that wider-ranging studies involving both conditions are necessary, with a prolonged program of supervised exercise training.
Cunha et al.38 assessed the role of the ANS in the pathogenesis of CCC. They analyzed 75 patients divided into four groups based on the Los Andes classification (IA, IB, II and III) and measured norepinephrine in 24-h urine in 52 patients with CCC (69%), 12 with other forms of heart disease (in NYHA class IV), the control group, and ten normal individuals. They performed 24-h Holter monitoring in 56 patients (74.6%) to assess HRV, the parameters being analyzed and distributed according to the Los Andes and Lown classifications. Norepinephrine levels were significantly higher (p=0.0001) in the controls (patients with non-Chagas heart disease) than in group III (CCC patients with heart failure). Reduced HRV was observed in CD patients, although this did not correlate with functional class. The authors concluded that norepinephrine levels in CD patients in an advanced stage of cardiac involvement (group III) do not rise as in other forms of heart disease with a similar degree of cardiac involvement, which probably reflects damage to the sympathetic nervous system. HRV was reduced, but one index of the parasympathetic system (daytime and nighttime rMSSD) was elevated in a group of patients with complex arrhythmias.
ConclusionThe HRV parameters SDNN, rMSSD and pNN50 in our patients with CCC did not undergo statistically significant changes after a six-month cardiac rehabilitation program.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Amaral da Silva Souza MV, et al. Variabilidade da frequência cardíaca: análise dos índices no domínio do tempo em portadores de cardiopatia chagásica crónica, antes e após um programa de exercícios. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.12.004.