Isolated left ventricular noncompaction (LVNC) is a rare cardiomyopathy characterized by excessive and prominent trabeculations associated with deep recesses that communicate with the ventricular cavity. Determining the natural history of this condition has been hampered by differences in clinical features and prognosis in published series, which are partly the result of differing diagnostic criteria and the lack of management guidelines. This work aims to contribute to the characterization of isolated LVNC by analyzing an affected population in terms of clinical presentation, diagnosis, risk stratification, treatment and follow-up. We also discuss the most relevant data from the literature concerning this cardiomyopathy.
A não compactação isolada do ventrículo esquerdo (NCIVE) é uma cardiomiopatia rara, caracterizada pela presença de trabeculações miocárdicas proeminentes e recessos intertrabeculares profundos que comunicam com a cavidade ventricular. A existência de diferentes critérios de diagnóstico, bem como a ausência de recomendações consensuais relativas à sua terapêutica, têm contribuído para que sejam descritas séries de doentes com características clínicas e prognósticos distintos, o que dificulta o reconhecimento da verdadeira história natural desta patologia. Este trabalho tem por objetivo contribuir para a caracterização da NCIVE, através da descrição de uma população afetada, no que se refere à sua apresentação clínica, diagnóstico, estratificação de risco, terapêutica instituída e seguimento. A propósito da revisão dos casos, é feita uma discussão da literatura atual mais relevante acerca do tema.
Isolated left ventricular noncompaction (LVNC) is a rare cardiomyopathy characterized by excessive and prominent trabeculations associated with deep recesses that communicate with the ventricular cavity but not with the coronary circulation.
Its real prevalence is unknown. Increasing awareness of the condition among physicians, together with advances in imaging techniques, have led to a growing number of reported cases, although it is still thought to be under-diagnosed.
The most common clinical manifestations are heart failure, ventricular arrhythmias and thromboembolic phenomena.
Diagnosis has traditionally been by echocardiography, but magnetic resonance imaging (MRI) is increasingly used.
Despite the growing interest in this cardiomyopathy, its natural history is poorly understood and the best therapeutic strategies have yet to be determined.
This work analyzes the population with LVNC treated in our center in terms of clinical presentation, diagnosis, risk stratification, treatment and follow-up. We also discuss the most relevant data from the literature concerning this cardiomyopathy.
MethodsWe retrospectively analyzed the medical records of patients diagnosed with LVNC and followed in outpatient cardiology consultations in our hospital up to December 2011.
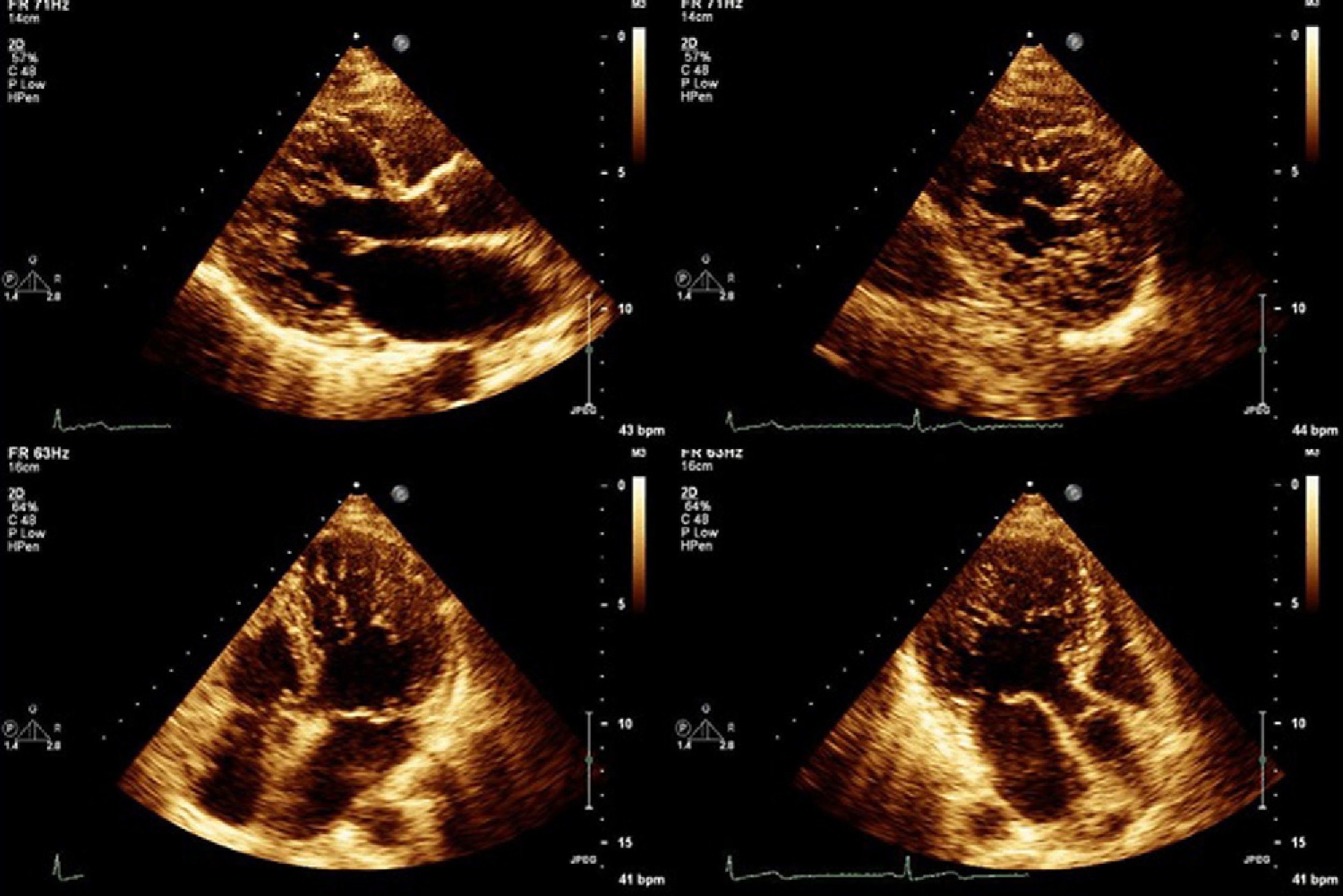
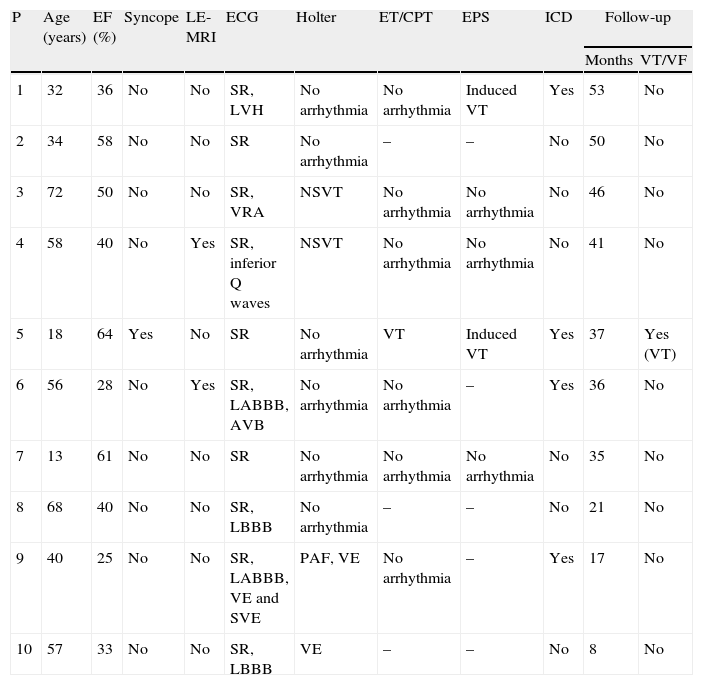
All patients were assessed by two-dimensional color Doppler and three-dimensional echocardiography using iE33 scanners (Philips Medical Systems). Contrast was used in one patient to exclude intraventricular thrombi. The diagnostic criteria of Jenni et al. were used: a ratio of non-compacted to compacted layers of >2 measured in systole; numerous prominent trabeculations and deep intertrabecular recesses filled with blood from the ventricular cavity, demonstrated by color Doppler; and absence of associated cardiac abnormalities.1
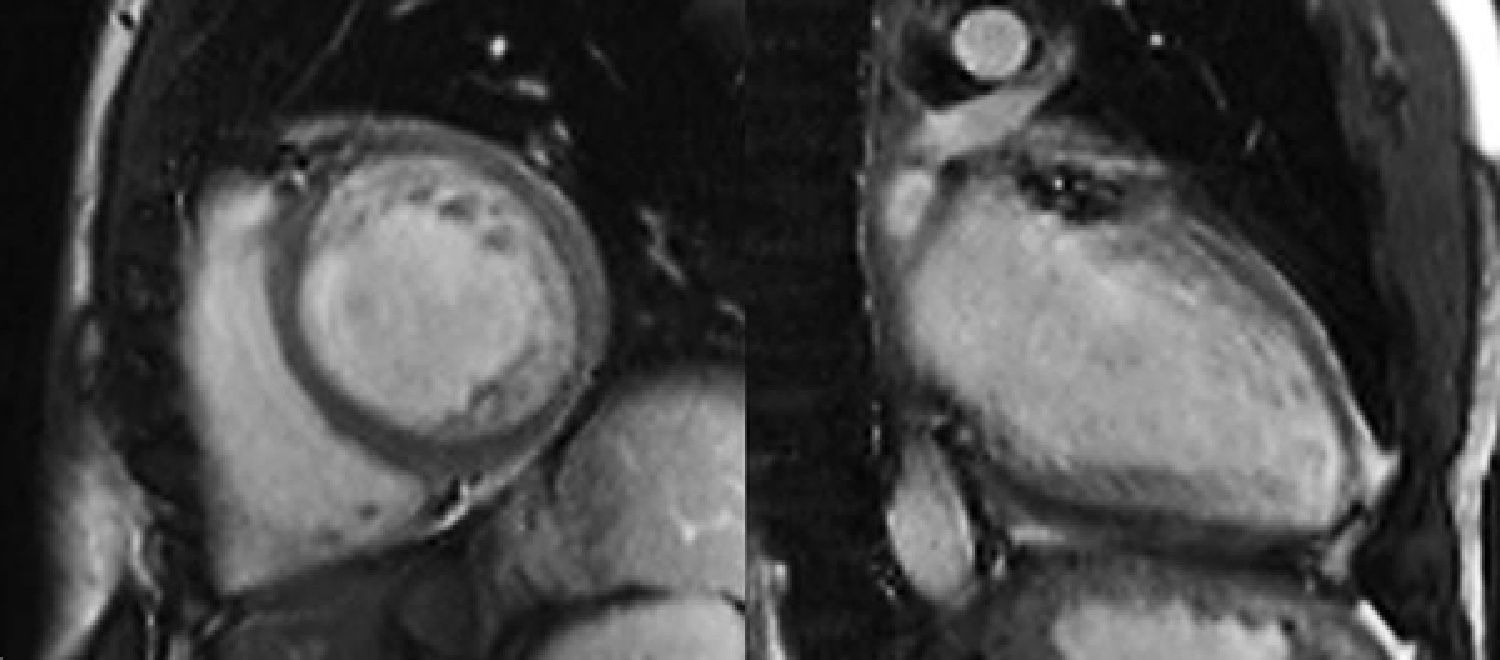
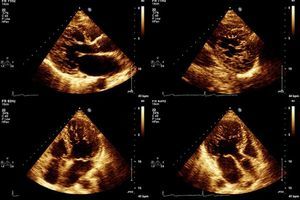
The diagnosis was confirmed in all patients by MRI on a 1.5-T Magnetom Symphony scanner (Siemens Medical Solutions) using Petersen et al.’s criterion: a ratio of end-diastolic thickness of non-compacted to compacted layers of >2.3.2
All patients underwent baseline electrocardiography (ECG) and Holter ambulatory monitoring. Exercise testing or cardiopulmonary testing were performed at the discretion of the attending physician.
Patients with impaired left ventricular (LV) systolic function were assessed by multidetector computed tomography (MDCT) or cardiac catheterization to exclude coronary disease.
Electrophysiological study (EPS) to stratify arrhythmic risk was performed at the discretion of the attending physician. The protocol for programmed electrical stimulation consisted of two basic cycles and two prolonged extrastimuli until the ventricular refractory period or a minimum coupling interval of 200ms.
Echocardiographic screening of first-degree relatives was recommended to all patients. Index patients with familial forms of LVNC underwent genetic study to screen for mutations in the TAZ and LDB3 genes.
Mean follow-up was 36.5 (8–53) months.
ResultsTen patients, all Caucasian, six male (60%), mean age 48 years (13–72), were diagnosed with LVNC between July 2007 and December 2011.
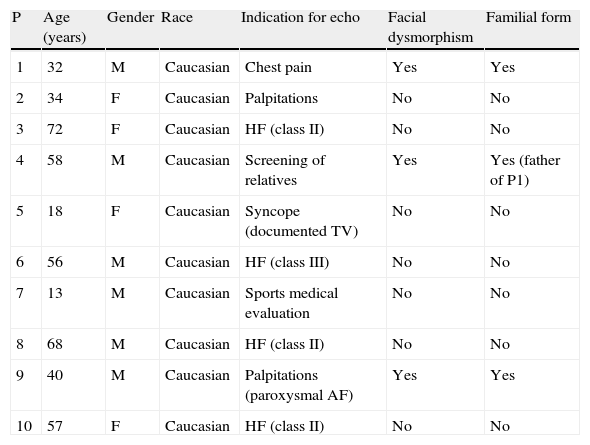
At the time of diagnosis, four patients had symptoms of heart failure: one in New York Heart Association (NYHA) class III and three in class II. There were different indications for echocardiography in the other patients (Table 1): an 18-year-old woman who reported episodes of syncope, with an episode of sustained monomorphic ventricular tachycardia (VT) during exercise testing; two patients with palpitations (one with documented paroxysmal atrial fibrillation [AF] and the other with no identified arrhythmia); one with atypical chest pain; a 13-year-old boy diagnosed following a sports medical evaluation; and one patient identified by screening of relatives of an index case.
Characteristics of the study population.
| P | Age (years) | Gender | Race | Indication for echo | Facial dysmorphism | Familial form |
| 1 | 32 | M | Caucasian | Chest pain | Yes | Yes |
| 2 | 34 | F | Caucasian | Palpitations | No | No |
| 3 | 72 | F | Caucasian | HF (class II) | No | No |
| 4 | 58 | M | Caucasian | Screening of relatives | Yes | Yes (father of P1) |
| 5 | 18 | F | Caucasian | Syncope (documented TV) | No | No |
| 6 | 56 | M | Caucasian | HF (class III) | No | No |
| 7 | 13 | M | Caucasian | Sports medical evaluation | No | No |
| 8 | 68 | M | Caucasian | HF (class II) | No | No |
| 9 | 40 | M | Caucasian | Palpitations (paroxysmal AF) | Yes | Yes |
| 10 | 57 | F | Caucasian | HF (class II) | No | No |
AF: atrial fibrillation; echo: echocardiography; F: female; HF: heart failure; M: male; TV: sustained ventricular tachycardia.
None of the patients had a family history of cardiomyopathy or sudden cardiac death.
Facial dysmorphism (high arched palate, low-set ears, and prominent forehead) was observed in three patients, all of whom had familial forms of LVNC. No neuromuscular disease was found (although it should be borne in mind that only three patients had been assessed by a neurologist).
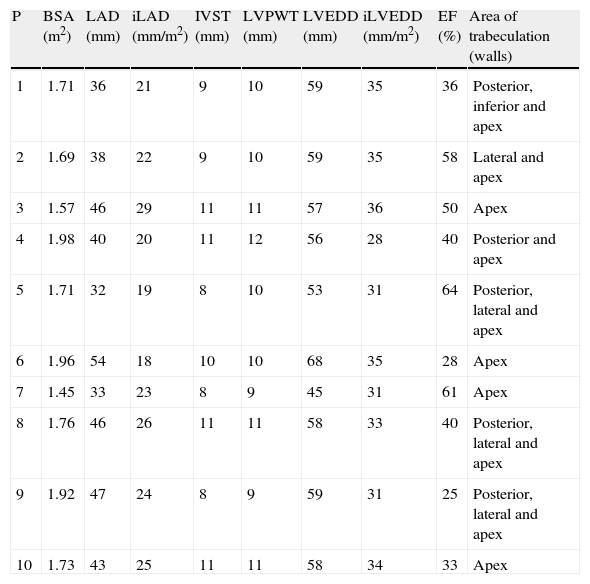
Echocardiographic study (Table 2) revealed LV dilatation in six patients (mild in two cases and moderate in the other four). Seven patients presented global LV dyskinesia with impaired systolic function (mild in one patient, moderate in four and severe in two). The region most frequently affected by noncompaction was the apex, followed by the posterior and lateral walls, mainly in the mid and apical segments. In two cases hypertrabeculation was also observed in the apex of the right ventricle (RV). Ventricular wall thicknesses were within normal limits or slightly increased. Five patients presented dilatation of the left atrium.
Echocardiographic characteristics of the study population.
| P | BSA (m2) | LAD (mm) | iLAD (mm/m2) | IVST (mm) | LVPWT (mm) | LVEDD (mm) | iLVEDD (mm/m2) | EF (%) | Area of trabeculation (walls) |
| 1 | 1.71 | 36 | 21 | 9 | 10 | 59 | 35 | 36 | Posterior, inferior and apex |
| 2 | 1.69 | 38 | 22 | 9 | 10 | 59 | 35 | 58 | Lateral and apex |
| 3 | 1.57 | 46 | 29 | 11 | 11 | 57 | 36 | 50 | Apex |
| 4 | 1.98 | 40 | 20 | 11 | 12 | 56 | 28 | 40 | Posterior and apex |
| 5 | 1.71 | 32 | 19 | 8 | 10 | 53 | 31 | 64 | Posterior, lateral and apex |
| 6 | 1.96 | 54 | 18 | 10 | 10 | 68 | 35 | 28 | Apex |
| 7 | 1.45 | 33 | 23 | 8 | 9 | 45 | 31 | 61 | Apex |
| 8 | 1.76 | 46 | 26 | 11 | 11 | 58 | 33 | 40 | Posterior, lateral and apex |
| 9 | 1.92 | 47 | 24 | 8 | 9 | 59 | 31 | 25 | Posterior, lateral and apex |
| 10 | 1.73 | 43 | 25 | 11 | 11 | 58 | 34 | 33 | Apex |
BSA: body surface area; EF: ejection fraction; iLAD: indexed left atrial diameter; iLVEDD: indexed left ventricular end-diastolic diameter; IVST: interventricular septal thickness; LAD: left atrial diameter; LVEDD: left ventricular end-diastolic diameter; LVWP: left ventricular posterior wall thickness; P: patient.
MRI study revealed late gadolinium enhancement in two patients (Table 3).
Characterization of arrhythmias in the study population.
| P | Age (years) | EF (%) | Syncope | LE-MRI | ECG | Holter | ET/CPT | EPS | ICD | Follow-up | |
| Months | VT/VF | ||||||||||
| 1 | 32 | 36 | No | No | SR, LVH | No arrhythmia | No arrhythmia | Induced VT | Yes | 53 | No |
| 2 | 34 | 58 | No | No | SR | No arrhythmia | – | – | No | 50 | No |
| 3 | 72 | 50 | No | No | SR, VRA | NSVT | No arrhythmia | No arrhythmia | No | 46 | No |
| 4 | 58 | 40 | No | Yes | SR, inferior Q waves | NSVT | No arrhythmia | No arrhythmia | No | 41 | No |
| 5 | 18 | 64 | Yes | No | SR | No arrhythmia | VT | Induced VT | Yes | 37 | Yes (VT) |
| 6 | 56 | 28 | No | Yes | SR, LABBB, AVB | No arrhythmia | No arrhythmia | – | Yes | 36 | No |
| 7 | 13 | 61 | No | No | SR | No arrhythmia | No arrhythmia | No arrhythmia | No | 35 | No |
| 8 | 68 | 40 | No | No | SR, LBBB | No arrhythmia | – | – | No | 21 | No |
| 9 | 40 | 25 | No | No | SR, LABBB, VE and SVE | PAF, VE | No arrhythmia | – | Yes | 17 | No |
| 10 | 57 | 33 | No | No | SR, LBBB | VE | – | – | No | 8 | No |
AVB: first-degree atrioventricular block; CPT: cardiopulmonary testing; ECG: electrocardiogram; EF: ejection fraction; EPS: electrophysiological study; ET: exercise testing; ICD: implantable cardioverter-defibrillator; LABBB: left anterior bundle branch block; LBBB: complete left bundle branch block; LVH: criteria for left ventricular hypertrophy; LE-MRI: late enhancement magnetic resonance imaging; NSVT: nonsustained ventricular tachycardia; PAF: paroxysmal atrial fibrillation; SR: sinus rhythm; SVE: supraventricular extrasystoles; VE: ventricular extrasystoles; VF: ventricular fibrillation; VRA: ventricular repolarization abnormalities; VT: ventricular tachycardia.
Of the seven patients who underwent coronary angiography by MDCT or cardiac catheterization, only one had coronary disease (a single 70% lesion in the proximal circumflex artery). This patient underwent myocardial perfusion scintigraphy which revealed no perfusion defects at rest or during exercise, and so the coronary disease detected was judged insufficient to explain the patient's LV dilatation and impaired function.
The baseline ECG showed alterations in seven patients (Table 3), the most common of which were left ventricular conduction disturbances (complete left bundle branch block and left anterior bundle branch block). Other ECG findings included ventricular repolarization abnormalities, criteria for LV hypertrophy, first-degree atrioventricular block and pathological Q waves.
Holter ambulatory monitoring revealed frequent ventricular extrasystoles in two patients and isolated short runs of nonsustained ventricular tachycardia (NSVT) in two others. One patient presented periods of paroxysmal AF.
Four patients underwent cardiopulmonary testing, which showed normal functional capacity in all cases, with a median peak VO2 of 36ml/kg/min (21–53) and attainment of a median of 116% of the predicted value (90–123). Three of these patients had impaired LV systolic function. Three other patients underwent exercise testing, with good exercise tolerance and no arrhythmias.
Five patients underwent EPS for arrhythmic risk stratification (Table 3). Of the others, two were referred directly for implantation of a cardioverter–defibrillator (ICD) due to severe LV dysfunction, and three did not undergo EPS, two on the decision of the attending cardiologist and one because the patient refused. After programmed electrical stimulation according to the predetermined protocol, polymorphic VT with syncope was induced in two patients (one with documented spontaneous sustained monomorphic VT, the other with no documented arrhythmias and asymptomatic, but with moderate ventricular dysfunction). No arrhythmias were induced in the other patients.
Echocardiographic screening of first-degree relatives was positive in two cases: the father of one index patient, who is included in our series, and the daughter of another index patient, then seven years old, who was referred to a pediatric cardiologist and was therefore excluded. Screening was incomplete in some families due to the refusal of some members.
Genetic study in the two index patients with familial forms of LVNC was negative in one and is still in progress in the other.
Oral anticoagulation therapy was prescribed for all patients with moderate or severe LV systolic dysfunction and/or AF. All the others, except for the 13-year-old boy, were prescribed aspirin. All received state-of-the-art HF treatment. Four patients received ICDs: one with spontaneous VT inducible on EPS, for secondary prevention; and three for primary prevention (two with severe LV dysfunction and one with VT inducible on EPS). The other patients remained under clinical surveillance with periodic Holter monitoring.
Survival after a median follow-up of 36.5 (8–53) months was 100%. Patients who had been asymptomatic at diagnosis remained so, and three of the four patients with HF remained stable (in class II) and one improved with treatment (from class III to class II). Serial echocardiography showed no worsening of LV dysfunction, and no thromboembolic phenomena were recorded. One appropriate shock was documented at 32 months of follow-up in the patient with an ICD for secondary prevention. No shocks, appropriate or inappropriate, were recorded in the patients with ICDs for primary prevention, although brief periods of asymptomatic NSVT were documented in all of them. No VT was recorded in any of the patients under clinical surveillance.
DiscussionAlthough our experience with LVNC is relatively recent and limited, we decided to publish in order to contribute to the characterization of this cardiomyopathy in a Portuguese population. Our series is somewhat heterogeneous in terms of how LVNC was diagnosed and managed, which reflects the lack of universally accepted guidelines for this condition. We therefore present a review of recent studies on LVNC.
“Persisting sinusoids” in the ventricular myocardium were first described in association with congenital heart disease with ventricular outflow tract obstruction or semilunar valve atresia.3–5 It was suggested that in these cases intraventricular pressure overload prevented the regression of embryonic myocardial sinusoids, resulting in intertrabecular recesses communicating with the ventricular cavity and the coronary circulation. The first report of “persistence of isolated myocardial sinusoids” was by Engberding and Bender in 1984, who described this morphology in the absence of other cardiac defects that might explain the abnormal myocardial development.6 The term “isolated noncompaction of left ventricular myocardium” was coined by Chin et al., who suggested that the probable etiological mechanism was an arrest of the normal process of compaction of the myocardium.7 During early embryonic development, the myocardium is a spongy network of interwoven fibers forming trabeculae and separated by intertrabecular recesses that communicate with the left ventricular cavity, which provides its blood supply. Gradual compaction of the myocardium occurs between weeks 5 and 8 of embryonic life, proceeding from the epicardium to endocardium and from the base of the heart to the apex. The coronary circulation develops concurrently, and the intertrabecular recesses are reduced to capillaries. A genetically determined arrest of this process has been put forward as the mechanism behind LVNC, but cases have been described of apparently acquired LVNC, particularly in association with neuromuscular disease, which suggests that genetics may not be the only factor involved.8–11 However, even in such cases, the noncompaction phenotype may develop in response to stimuli in genetically predisposed individuals only or in a particular type of myocardial structure.12
LVNC was included among the “unclassified cardiomyopathies” by the World Health Organization,13 but more recently has been classified as a primary congenital cardiomyopathy by the American Heart Association.14
The normal process of compaction is more complete in the LV than in the right ventricle (RV), with the result that the latter has a more trabeculated appearance. It is thus more difficult to distinguish between normal and pathological forms of RV noncompaction, and several authors have chosen to restrict the diagnosis to LV noncompaction.1,15
The actual prevalence of LVNC is unknown; it is usually considered to be underdiagnosed. Observational studies report a prevalence of less than 0.14% in adults referred for echocardiographic study.15,16
In our series, age at diagnosis ranged between 13 and 72 years, and most patients were male. Although initially described as a rare condition affecting children, several cases have been reported of presentation later in life. Various reasons have been proposed for the predominance of males12,17: X-linked heredity; the possibility that females are more severely affected and thus have higher early mortality (which would explain the predominance of females in some pediatric series), or that men are more liable to have acquired forms; and possible selection bias in favor of males in referral for echocardiography. It should also be borne in mind that the current diagnostic criteria do not take gender into account. A recent study showed differences between the sexes in the location and extent of noncompaction, with women presenting a larger area mainly with involvement of the anterior, lateral and posteroinferior walls, while in men the area is smaller and mainly apical. However, these differences are not reflected in different clinical characteristics or prognosis.17
A much debated issue is the lack of universally accepted diagnostic criteria. Transthoracic echocardiography has been the exam of choice for diagnosis of LVNC, but other techniques, including transesophageal, three-dimensional and contrast echocardiography, can also help to define the endocardial border and to rule out involvement of the chordae tendineae, papillary muscles and muscle bands. Besides Jenni's criteria, which were used in this study (see Methods), other echocardiographic criteria have been put forward. Chin et al. suggest an X-to-Y ratio of ≤0.5, where X is the distance between the epicardial surface and the trough of a trabecular recess, and Y is the distance between the epicardial surface and the peak of the trabeculation, measured at end-diastole.7 According to Stöllberger et al., the diagnosis is established when there are more than three trabeculations protruding from the LV wall, apically to the papillary muscles, visible in a single image plane, and intertrabecular spaces perfused from the ventricular cavity visualized on color Doppler imaging.18
In 2008, Kohli et al. demonstrated that there was a poor correlation between the above three echocardiographic definitions and that they were too sensitive, especially in blacks. They also raised the possibility that LVNC is being overdiagnosed, thus subjecting normal individuals to unnecessary and possibly harmful investigations and treatment, and it is thus urgent to determine the limits of normal trabecular patterns for different races and both sexes.19
In our center we follow the criteria of Jenni et al., as they are the most commonly used. However, as pointed out by several authors,20,21 it can be difficult to obtain rigorous and reproducible measurements of the thickness of the compacted and noncompacted layers, and so all patients also underwent MRI to confirm the diagnosis. This technique, as well as showing good agreement with echocardiographic findings, also has superior spatial resolution that enables better visualization of the LV apex and lateral wall, which are often involved in noncompaction. We consider that images from MRI are particularly suitable for quantitative assessment, for which the standard criteria are those of Petersen et al., as used in this study (see Methods). In 2010, Jacquier et al. showed that measurement of trabeculated LV mass by MRI can be used in the diagnosis of LVNC. According to these authors, a value above 20% of the total mass of the LV has high sensitivity and specificity for the diagnosis of LVNC (Figures 1 and 2).22
Another advantage of MRI is its contribution to arrhythmic risk stratification by identifying foci of subendocardial fibrosis by late gadolinium enhancement. In our series, late enhancement was only observed in two patients, neither of whom presented malignant ventricular arrhythmias.
Differential diagnosis is mainly with apical hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM). A review of published cases of LVNC misdiagnosed on initial echocardiographic assessment found initial diagnoses of endomyocardial fibroelastosis, restrictive cardiomyopathy, myocarditis, LV thrombus, cardiac metastases, aberrant chordae tendineae, and intramyocardial abscess or hematoma, as well as HCM and DCM.23 There have also been reports of pronounced hypertrabeculation in competitive athletes.12
The role of genetics in the diagnosis of LVNC is as yet unclear. Many cases are sporadic, but over half are familial.12 In our series, screening of first-degree relatives, although incomplete, was positive in two of nine index cases (22%). As in other cardiomyopathies, LVNC is genetically heterogeneous, with multiple mutations and forms of transmission having been described. The most common form of inheritance is autosomal dominant, but cases of X-linked and mitochondrial inheritance have also been reported.24,25 There is phenotypic variability within families and low penetrance, with some family members sharing the same mutation but expressing subtle or asymptomatic forms.26 Some of the mutations found had already been described in neuromuscular disease and other cardiomyopathies, especially HCM and DCM, and LVNC patients often have associated neuromuscular disease, while HCM and DCM may be found in their relatives.27 This genotypic and phenotypic overlapping has led some authors to suggest that rather than three distinct entities, LVNC, HCM and DCM are a continuum of cardiomyopathies.28 Further studies will be required to clarify the role of genetics in the diagnosis and prognosis of these patients, as well as in the monitoring of their relatives.
The most frequent clinical presentation in our population was HF, as in the largest published series.15,16,29–31 Facial dysmorphism was observed in three patients, two of them first-degree relatives and the third also with a familial form of LVNC. Curiously, facial dysmorphism has mainly been reported in pediatric series7; adult populations usually have a much lower prevalence of facial dysmorphism or none at all, which suggests that they may not have been systematically investigated. No neuromuscular disease was observed in our patients, although this association is so common that some authors recommend that all LVNC patients be assessed by a neurologist.18
Echocardiographic study revealed systolic dysfunction in seven patients (70%), which is in agreement with other studies.32 Although LV size was above normal in six patients, no severe dilatation was seen. This finding, which is also in agreement with the literature, is an important factor in differential diagnosis with DCM, which is usually characterized by greater LV dilatation, together with more severe systolic dysfunction and left atrial dilatation.33 The apical segments were most often affected by noncompaction, followed by the mid segments of the posterior and lateral walls. This distribution, which is also found in other series, may be related to the abnormal embryonic development referred to above.
Of the seven patients with LV dysfunction, only one presented coronary disease, and this was not responsible for his ventricular dysfunction. Several studies using positron emission tomography have shown reduced coronary reserve in LVNC patients, presumably secondary to microvascular dysfunction.34 Detection of subendocardial perfusion defects on MRI and observation in post-mortem studies of foci of subendocardial fibrosis lend support to the hypothesis that abnormalities in coronary microcirculation may play a part in contractile dysfunction and arrhythmogenesis.35,36 It has been suggested that altered perfusion and coronary flow reserve may be related to failure of the coronary microcirculation to grow with the increasing ventricular mass or to compression of the intramural coronary bed by the hypertrophied myocardium.37 The marked trabeculation of the LV may also impair diastolic function by causing abnormal relaxation and restrictive filling.38
The ECG is abnormal in most patients with LVNC, the most frequent alterations being left ventricular conduction disturbances,15 which was also found in our series.
VT was documented in three patients (30%): two episodes of NSVT on Holter monitoring in two patients, and one episode of sustained monomorphic VT on exercise testing in another. These findings are in line with other studies, in which the prevalence of VT (sustained or nonsustained) ranges between 20% and 47%.15,16,29 By contrast, in an Italian series of 238 patients (adults and children), VT was documented in only 4.6% (sustained in 0.8%) in a mean follow-up of four years.39 However, sudden death is one of the leading causes of death in LVNC, occurring in up to 18% of affected individuals.15 This supports the idea that noncompacted myocardium can be a highly arrhythmogenic substrate. The mechanisms proposed include concomitant arrest of development of the conduction system or progressive ischemia due to hypoperfusion of the trabeculae. The deep intertrabecular recesses may themselves be a substrate for the propagation of reentry circuits underlying scar tissue.15
Stratification of arrhythmic risk in these patients is extremely difficult, the more so since malignant ventricular tachyarrhythmias have been documented in patients with preserved ventricular function. Periodic Holter monitoring is useful but insufficient. Exercise testing is important, particularly in patients with symptoms suggestive of exercise-induced arrhythmias, although routine testing is not recommended by most authors. The role of EPS in the management of LVNC patients is still unclear, and it is not included in the European and American guidelines for the management of ventricular arrhythmias and prevention of sudden cardiac death.40 Kobza et al. retrospectively studied 12 LVNC patients with ICDs (seven of whom had undergone EPS due to symptoms suggestive of arrhythmia or LV dysfunction, the others having received ICDs due to documented VT). The three patients in whom sustained VT or ventricular fibrillation (VF) had been induced during EPS received appropriate shocks, while in the other four patients, in whom no arrhythmia (or only NSVT) had been induced by programmed electrical stimulation, there was only one appropriate shock, in a patient with induced NSVT.41 Steffel et al. performed a retrospective analysis of 24 LVNC patients who underwent EPS on the decision of the attending physician. Programmed electrical stimulation induced VT or VF in four patients and NSVT in five. During a mean follow-up of five years, appropriate ICD shocks were recorded in three out of nine patients with inducible arrhythmias on EPS (two with VT or VF and one with NSVT). In the 15 patients with negative EPS, no episodes of ventricular tachyarrhythmia or sudden cardiac death were observed, but the follow-up was shorter (mean 2.5 years) and three patients were lost to follow-up. In view of these findings, the authors suggest that a negative EPS could identify patients at low risk of malignant VT, although the study's limitations (small sample size and different follow-up periods in the two groups) mean that conclusions cannot be drawn concerning the negative predictive value of EPS in LVNC patients. It should also be noted that these authors found no clinical, ECG or echocardiographic features that predicted inducibility of VT on EPS.42
Only five patients in our series underwent EPS, in two of whom polymorphic VT with syncope was induced. A less aggressive electrical stimulation protocol was chosen in order to identify patients with a low induction threshold for tachyarrhythmias. Four patients received an ICD, but only one appropriate therapy was recorded, in the patient with an ICD for secondary prevention.
In the study by Kobza et al., appropriate therapies were observed in 50% of patients treated for secondary prevention and in 25% of those treated for primary prevention.41 Caliskan et al., in a study of 44 LVNC patients with ICDs, reported appropriate shocks in 33% of patients treated for secondary prevention and in 13% of those treated for primary prevention.43
Although recent guidelines allow for the use of ICDs for primary prevention in LVNC patients (class IIb recommendation, level of evidence C),44 current thinking among the majority of authors is to follow the guidelines established for primary and secondary prevention in nonischemic cardiomyopathies.42,43
Treatment of HF should also follow current guidelines. In cases of severe LV dysfunction and left bundle branch block, cardiac resynchronization therapy (CRT) should be considered. A recent study comparing ventricular reverse remodeling following CRT in patients with LVNC and those with DCM revealed that the percentage of super-responders was significantly higher in the LVNC group, and that the larger the area of noncompaction, the greater the degree of reverse remodeling.45 Heart transplantation should be considered in patients with refractory HF.
Another important aspect of treatment in these patients is prevention of thromboembolism. The deep intertrabecular recesses of LVNC are conducive to pooling of blood and hence the formation of intraventricular thrombi. The first series of LVNC patients reported a high prevalence of thromboembolic events (24–38%), which led the authors to recommend oral anticoagulation for all patients.7,15 However, in more recent studies the rates of thromboembolism have been much lower (4–9%), which may reflect the greater number of anticoagulated patients in these series (up to 60%).16,29 Most authors now recommend oral anticoagulation only for patients with severe LV dysfunction, AF, intraventricular thrombi or a history of systemic thromboembolism.16,46,47 No thromboembolic events were recorded in our series and no intraventricular thrombi were detected. All patients with moderate to severe systolic dysfunction or AF were prescribed oral anticoagulants.
There is still uncertainty regarding the prognosis of LVNC patients. In one of the first series published, by Oechslin et al., 47% of patients died or were transplanted at 44 months,15 while Murphy et al. reported 2% mortality at 46 months.29 The more favorable prognosis in more recent series may be due to more aggressive treatment of patients with HF, arrhythmias and thromboembolism. At the same time, increasing awareness of LVNC among physicians, together with advances in imaging techniques, have resulted in a wider spectrum of LVNC patients being diagnosed, including asymptomatic forms, which were not included in early series.32 Factors leading to worse prognosis include greater LV diastolic diameter on initial assessment, NYHA class III or IV, permanent AF and left bundle branch block.15 High-risk patients should be considered for more aggressive treatment, including ICDs and evaluation for transplantation.
ConclusionsAlthough it is rare, recent years have seen considerable growth in the number of studies on LVNC. However, determining the natural history of this condition has been hampered by the lack of large series with long follow-up, while the use of differing diagnostic criteria and therapeutic approaches may partly explain the differences in the results of published series. The establishment of an international registry would help bring together these disparate sources of information, with a view to producing universally accepted guidelines for the diagnosis and treatment of these patients.
To summarize, the current state of knowledge is that LVNC is a rare cardiomyopathy whose main clinical manifestations are HF, arrhythmias and thromboembolism. Diagnosis has traditionally been by echocardiography, but MRI is increasingly used. The prognosis of advanced LVNC is poor, but can be improved by early diagnosis, systematic screening of affected families and aggressive treatment in high-risk patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sousa O, et al. Não compactação isolada do ventrículo esquerdo: experiência de um centro. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.06.010.