Distinguishing between zventricular aneurysm and pseudoaneurysm, although difficult, is of major importance due to the therapeutic and prognostic implications. The present case highlights the pivotal role of non-invasive imaging modalities for differential diagnosis between these entities in order to ensure appropriate management of these patients.
O diagnóstico diferencial entre o aneurisma e o pseudoaneurisma ventricular, embora difícil, é fundamental face às implicações terapêutica e prognóstica. O presente caso clínico realça o papel fulcral das técnicas de imagem não invasivas no diagnóstico diferencial destas entidades, possibilitando uma correta orientação dos doentes.
The advent of early myocardial revascularization has led to a reduction in the incidence of mechanical complications after myocardial infarction (MI). Nevertheless, left ventricular (LV) free wall rupture, one of the most feared complications, occurs in 4% of MI patients, and is responsible for around a quarter of related deaths.1 In rare cases, the rupture is contained by adherent pericardium, giving rise to a cavity delineated by scar tissue but with no muscle fibers, producing what has been termed a pseudoaneurysm; the risk of rupture is thus high2 and urgent surgical repair is necessary. Given the prognostic and therapeutic implications, prompt diagnosis is essential. However, there are no features of clinical presentation, physical examination, chest X-ray or electrocardiogram (ECG) that are sensitive and specific to ventricular pseudoaneurysms as opposed to true aneurysms, which are a more common complication of MI. The present case illustrates these difficulties in diagnosis and highlights the role of imaging techniques in identifying this entity.3
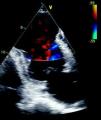
Case reportWe report the case of a 72-year-old man, white, an ex-smoker, with a history of transurethral prostatectomy and cerebrovascular disease. He was not taking any cardiovascular medication. In February 2009, he suffered prolonged crushing chest pain radiating to the back accompanied by vomiting, but did not seek medical attention. He then began experiencing heart failure symptoms, with progressively worsening exertional dyspnea, but without recurrence of chest pain. Approximately one month later, he came to the emergency department of our hospital due to worsening symptoms, and was found to be in New York Heart Association (NYHA) class IV. The admission ECG showed signs of a previous anterior MI; no elevation of myocardial necrosis markers was observed. Echocardiographic assessment revealed severe LV systolic dysfunction, an apical aneurysm with intense auto-contrast (Figure 1) and a sessile thrombus; oral anticoagulation was therefore initiated.
Due to suspicion of pulmonary tuberculosis and marked deterioration in the patient's general condition, non-invasive stratification was the initial approach adopted. Further studies during hospitalization in the internal medicine department revealed no microbiological agent in bronchial secretions, gastric juice or blood cultures. There was a progressive fall in markers of systemic inflammation, obviating the need for empirical antibiotic therapy. The patient was discharged three weeks later, and referred for outpatient consultation. Some months later, he was rehospitalized for worsening heart failure.
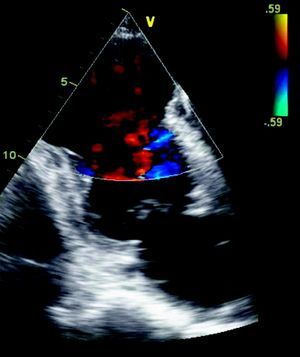
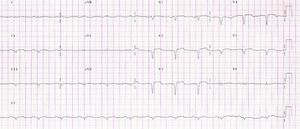
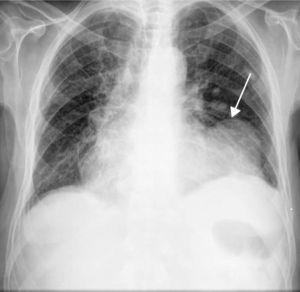
The ECG showed signs of a previous MI (Figure 2) and the chest X-ray revealed a mass adjacent to the cardiac silhouette (Figure 3). Repeat echocardiography showed a large apical aneurysm, the image being compatible with a pseudoaneurysm, extending infero-posteriorly and compressing the right ventricle (Figure 4).
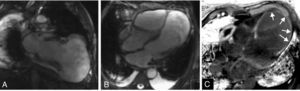
These findings prompted reversal of oral anticoagulation and suspension of antiplatelet therapy. Cardiac magnetic resonance imaging (CMRI) was performed to clarify the anatomy and aid the planning of surgical repair, which confirmed the presence of a large pseudoaneurysm and showed its extension and close relation to the right ventricle, which was subject to significant compression. Delayed enhancement study was able to define the extent of the infarct and documented the presence of viable myocardium in the mid-basal segments of the left ventricle (Figure 5).
Cardiac magnetic resonance imaging, steady-state free precession sequences in vertical long-axis (A) and 4-chamber (B) views, confirming the presence of a large left ventricular pseudoaneurysm. Delayed enhancement images (phase-sensitive inversion recovery) acquired 10minutes after administration of gadolinium, in 4-chamber view (C), show a transmural area of contrast uptake surrounding the aneurysm (arrows).
Following coronary angiography that showed occlusion of the mid segment of the anterior descending and 60% stenosis of the right coronary artery, the pseudoaneurysm was surgically resected, the LV aneurysm was excluded and the ventricle was reconstructed (Dor procedure) (Figure 6).
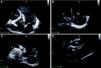
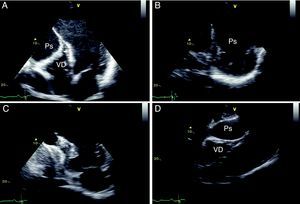
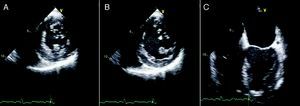
The patient's recovery was initially slow, but following discharge he has remained clinically stable, in NYHA class II. Repeat echocardiography three months after the surgical intervention showed normal LV dimensions, mildly impaired global systolic function, and a correctly positioned ventricular patch (Figure 7).
Two-dimensional transesophageal echocardiography, short-axis views in systole (A) and diastole (B) and 4-chamber view in diastole (C), showing a left ventricle of normal dimensions, mildly impaired systolic function and a correctly positioned ventricular patch, with no evidence of leakage.
Mechanical complications after MI are now much less frequent following implementation of effective early revascularization strategies. Although infrequent, cardiac rupture is one of the most feared events since it is almost always fatal. In rare cases, the rupture may be contained by adherent pericardium or scar tissue, giving rise to a saccular formation with no myocardial fibers, which is termed a pseudoaneurysm. Given the composition of its wall, there is a high risk of expansion and rupture,2 and urgent surgical repair is thus required. By contrast, a true aneurysm represents extreme maladaptive remodeling following an ischemic event. It consists of an area of thinned ventricular wall, still with three layers, that moves dyskinetically but has a low risk of rupture; it is therefore usually treated conservatively.
Occasionally, the two entities coexist,4,5 or a ventricular aneurysm can be complicated by rupture,3,6 as may have occurred in the case presented. Given the prognostic and therapeutic implications, a correct and prompt diagnosis of pseudoaneurysm is essential.
From a clinical standpoint, patients may be asymptomatic (up to 48% of cases7) or present with recurrent chest pain, signs of heart failure, syncope or thromboembolic phenomena.2,8 Sudden death is the form of presentation of ventricular pseudoaneurysm in only 3% of cases.8
Physical examination is of little value, usually only showing soft heart sounds, pericardial friction rub or de novo murmurs.
There are ECG alterations in most cases, with pathological Q waves or persistent ST-segment elevation in the infarct-related leads. In more than half of cases, the chest X-ray shows cardiomegaly and/or a mass adjacent to the cardiac silhouette, as seen in Figure 3. Nevertheless, while common, these findings are not specific, and cannot identify a pseudoaneurysm or differentiate between this and a true ventricular aneurysm. Cardiac imaging modalities thus play a pivotal role in characterizing this entity.
Transthoracic echocardiography, a readily available non-invasive imaging technique, is commonly used for the initial assessment of patients with MI, and helps not only with diagnosis, but also with determining the location and extent of the infarct, identifying mechanical complications and providing information that helps in stratifying risk and prognosis. Nevertheless, differential diagnosis between ventricular pseudoaneurysms and true aneurysms based on echocardiographic findings is a challenge. Inferior, posterior or lateral location,2 a ratio of <0.5 between the width of the neck and the maximal internal diameter of the aneurysmal sac,9 or the presence of bidirectional turbulent flow through the neck by color and pulsed Doppler study,10 are all suggestive of pseudoaneurysm, but such findings are limited in terms of sensitivity and specificity.2,3 According to Frances et al., in a series of 290 patients with ventricular pseudoaneurysm, transthoracic echocardiography enabled a definitive diagnosis in up to a third of cases.2 Diagnostic accuracy can be improved by using transesophageal echocardiography (accuracy of over 75%) or contrast agents that enable enhanced endocardial border delineation and identification of distortion of normal ventricular geometry such as a pseudoaneurysm.11
Left ventriculography is considered the gold standard imaging modality, with diagnostic accuracy of around 85%.2 The characteristic angiographic features of ventricular pseudoaneurysm are a narrow-necked aneurysmal sac with no adjacent coronary vessels in which contrast liquid remains for several cardiac cycles after injection.12 Furthermore, cardiac catheterization enables detection and characterization of associated coronary disease, valve disease (particularly mitral), and pulmonary hypertension, and thus helps in planning surgical treatment. However, it is an invasive technique that exposes the patient to ionizing radiation and presents a real risk of possible embolization of thrombotic material.
Recent advances have enabled computed tomography (CT) to be used for non-invasive coronary assessment as well as the acquisition of three-dimensional anatomical and functional information on the myocardium and pericardium. An interruption in the continuity of the endocardial outline, resulting in a narrow-necked aneurysmal sac with pulsatile flow, indicates a diagnosis of ventricular pseudoaneurysm.13 However, the fact that CT is not readily available, has limited temporal resolution, requires the use of iodinated contrast and exposes the patient to ionizing radiation, makes the technique a second-line option in this context.14
CMRI has been used since 1991 to improve diagnosis of ventricular pseudoaneurysms.15,16 This technique has high spatial resolution and the ability to characterize tissue, thus enabling non-invasive identification of the pericardium and the presence of thrombi, and can distinguish between necrotic and normal myocardium, which is not always possible with other imaging modalities. Besides providing information on overall morphology and function, particularly ventricular volumes and systolic and valve function, CMRI provides better morphological definition of a pseudoaneurysm's location, extension and its relations to adjacent structures. Moreover, delayed enhancement sequences enable accurate assessment of the location and extent of the infarcted area and of viable myocardium, thus contributing to pre-operative planning. Pericardial delayed enhancement (not only bordering the false cavity but in areas surrounding normal myocardium) has been proposed as a useful method of distinguishing between pseudoaneurysm and true ventricular aneurysm, with a sensitivity of 100% and specificity of 83%.17 It is considered to reflect pericardial inflammation and fibrosis arising from the seepage of blood into the pericardial space at the time of rupture.16
Given its many advantages, CMRI has great appeal as an imaging modality, with enormous potential to differentiate between ventricular aneurysms and pseudoaneurysms. In view of its growing availability and advances in acquisition sequences, the technique is increasingly used in clinical practice, even in relatively unstable patients, as in the case presented. It has added diagnostic value over echocardiography, particularly in patients with poor image quality, and is able to accurately assess the extent of the infarcted area and the number of viable segments, as well as to determine the relations of the pseudoaneurysm to the mitral valve and papillary muscles. Such an assessment is crucial to planning the surgical technique to adopt, which in most cases will be conventional surgical repair, but can mean heart transplantation in selected cases.
ConclusionsDifferential diagnosis between aneurysm and pseudoaneurysm is particularly difficult but of major importance due to the therapeutic and prognostic implications. Improvements in the resolution of non-invasive cardiac imaging modalities have contributed to more accurate and prompt diagnoses, ensuring appropriate management of these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Oliveira S, et al. Pseudoaneurisma gigante do ventrículo esquerdo: contributo diagnóstico de diferentes modalidades de imagem não invasivas. Rev Port Cardiol. 2012. doi:10.1016/j.repc.2012.04.009.