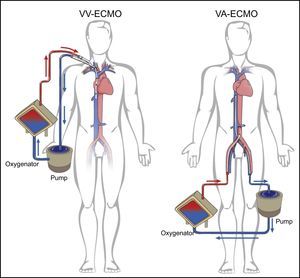
Extracorporeal membrane oxygenation (ECMO) is being increasingly used for short-term circulatory and/or respiratory support. It is derived from extracorporeal circulation systems used in cardiac surgery in which blood is propelled by a centrifugal pump through an external membrane where gas exchange takes place. The pump assists the heart and the membrane fulfills the functions of the lungs.1,2 ECMO is indicated in cases of respiratory failure such as acute respiratory distress syndrome, or cardiac failure such as myocardial infarction complicated by cardiogenic shock, which although acute carry the possibility of recovery, and in patients with advanced chronic heart failure as a bridge to transplantation or decision.3–5 Circulation is usually established via peripheral venous and/or arterial cannulation, increasingly by a percutaneous approach. There are two basic types (Figure 1): veno-venous (VV), for respiratory support; and venoarterial (VV), for circulatory and/or cardiopulmonary support.6,7
Modalities of extracorporeal membrane oxygenation. VA: venoarterial, for circulatory and/or respiratory support; VV: venovenous, for respiratory support. Adapted from Squiers et al.2
ECMO is a highly effective and low-cost technique (the console, a one-off purchase, costs around fifty thousand euros and the consumables around five thousand euros per patient) that can be used in any hospital, not necessarily tertiary. Modern systems are portable and thus enable patients to be transferred between hospitals.8–10 However, although the system is relatively easy to use and to monitor even for those without a high degree of technical knowledge, ECMO should only be performed by physicians who are trained and experienced in starting, maintaining and ending the treatment, and its use should be concentrated in specialist centers where a solid knowledge of respiratory and circulatory pathophysiology can render it most effective.
In this issue of the Journal, the cardiology group at Vila Nova de Gaia describe their experience with 48 patients who underwent ECMO, 26 of them VA-ECMO and 19 of them VV-ECMO, over a period of around five and a half years.11 This is probably the largest series published in Portugal, although larger ones have been published elsewhere. The study's results are in agreement with those in high-volume centers with considerable experience. Almost 70% of patients in both groups were successfully weaned from ECMO and survival to hospital discharge was 37.9% in patients undergoing VA-ECMO and 63.2% in those receiving VV-ECMO. Comparisons between these two groups is an interesting intellectual exercise but without real clinical significance, given the differences between the two techniques and the patients treated, in terms of both underlying disease and comorbidities. As expected, in the VA-ECMO group, the need for more inotropic drugs, an indication of greater myocardial dysfunction, was a predictor of mortality.
Naturally, a therapy of this kind is associated with not inconsiderable risks. There is a high incidence of thromboembolism due to the large area of contact between the blood and the membrane and tubes making up the system, and so at least some degree of anticoagulation is required, which increases the likelihood of bleeding complications, especially related to peripheral cannulation. Anticoagulation and the traumatic effects of pumping on blood cells can give rise to serious hematological dysfunction, including hemolysis and thrombocytopenia. There is also a risk of local and systemic infection.
Furthermore, blood circulation in ECMO is not physiologically pulsatile, especially in cases of severe myocardial dysfunction in which there is little or no ejection by the heart. This can result in inadequate perfusion of vital organs such as the brain, kidneys, liver and gastrointestinal tract, particularly if the therapy is prolonged. Finally, the cannulated peripheral vessels are prone to vascular complications, including ischemia of downstream territories. This is a common complication when the femoral artery is cannulated, and so a shunt is now routinely placed to maintain distal perfusion.
Besides the course of the underlying disease, these complications are themselves frequently a cause of death, thus seriously compromising medium- and long-term survival. Notwithstanding, it should be borne in mind that these patients would very likely not survive without such treatment.
Unfortunately, of the nine patients in the Gaia group's series who were referred for cardiac transplantation (one of the indications for ECMO), only three were transplanted and of these only one survived, the rest dying while awaiting a donor. The length of waiting lists and the time spent on them are due to the lack of donors. Although patients receiving mechanical ventricular assistance are always given top priority, and although the laws governing organ donation in Portugal are among the most liberal in the world (our donation rates are among the highest in Europe, around 30 donors per million population), often the new heart does not arrive in time. In my center, survival in transplanted patients with a ventricular assist device was around 70%, but mortality in those on ventricular assistance for more than two weeks at the time of transplantation was much higher than in those who were transplanted earlier.
In conclusion, ECMO is being used as a ventricular assist technique in several centers in Portugal, particularly those with on-site cardiac surgery facilities, which is to be expected given the specific applications of the technology. However, in principle it should be available for all patients in all the hospitals in the country, since the equipment is portable and so patients and supporting teams can move without undue risk from peripheral centers to tertiary hospitals. This occurred with many of the patients treated at the Gaia center, where almost half of patients undergoing VA-ECMO were transferred in cardiogenic shock from another hospital, but experience suggests that these patients might benefit from having the device placed in the hospital of origin, before being transferred.9 With growing experience and ever-improving results, the indications for this therapeutic modality are continuing to widen. Nevertheless, it is essential to avoid using ECMO in patients in whom it would be futile – those in the terminal stages of disease, those treated for too long by conventional means, and those diagnosed with a fatal condition.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Antunes MJ. Extra-Corporeal Membrane Oxygenation na insuficiência respiratória e circulatória agudas: uma terapêutica cada vez mais à mão. Rev Port Cardiol. 2017;36:843–845.