Extracorporeal membrane oxygenation (ECMO) provides mechanical pulmonary and circulatory support for patients with shock refractory to conventional medical therapy. In this study we aim to describe the indications, clinical characteristics, complications and mortality associated with use of ECMO in a single tertiary hospital.
MethodsWe conducted a retrospective observational cohort study of all patients supported with ECMO in two different intensive care units (general and cardiac), from the first patient cannulated in April 2011 up to October 2016.
ResultsOverall, 48 patients underwent ECMO: 29 venoarterial ECMO (VA-ECMO) and 19 venovenous ECMO (VV-ECMO). In VA-ECMO, acute myocardial infarction was the main reason for placement. The most frequent complication was lower limb ischemia and the most common organ dysfunction was acute renal failure. In VV-ECMO, acute respiratory distress syndrome after viral infection was the leading reason for device placement. Access site bleeding and hematologic dysfunction were the most prevalent complication and organ dysfunction, respectively. Almost 70% of ECMO episodes were successfully weaned in each group. Survival to discharge was 37.9% for VA-ECMO and 63.2% for VV-ECMO. In VA-ECMO, the number of inotropic agents was a predictor of mortality.
ConclusionPatients with respiratory indications for ECMO experienced better survival than cardiac patients. The need for more inotropic drugs was a predictor of mortality in VA-ECMO. This is the first published record of the overall experience with ECMO in a Portuguese tertiary hospital.
A oxigenação por membrana extracorporal (ECMO) permite o suporte mecânico em doentes com falência cardiovascular e/ou pulmonar. Neste estudo pretendemos descrever as indicações, características clínicas, complicações e mortalidade associadas ao uso de ECMO num hospital terciário.
MétodosFoi realizado um estudo de coorte retrospetivo e observacional de todos os doentes que implantaram ECMO, em duas unidades de cuidados intensivos (polivalente e cardíaca), desde o primeiro doente canulado em abril/2011 até outubro/2016.
ResultadosQuarenta e oito doentes colocaram ECMO: 29 ECMO venoarterial (ECMO-VA) e 19 ECMO venovenoso (ECMO-VV). No ECMO-VA, o enfarte agudo do miocárdio foi a principal indicação para a sua implantação. A complicação mais frequente foi a isquemia do membro inferior e a disfunção de órgão associada mais comum foi a renal. No ECMO-VV, a síndrome de dificuldade respiratória aguda secundária a infeção viral foi o motivo dominante para a utilização do dispositivo. A hemorragia pelo local de acesso e a disfunção hematológica foram, respetivamente, a complicação e a disfunção de órgão mais prevalentes. Foram descanulados com sucesso quase 70% dos doentes, em ambos os grupos. Os doentes em ECMO-VA tiveram sobrevivência hospitalar de 37,9% e os em ECMO-VV 63,2%. O número de agentes inotrópicos foi preditor de mortalidade no ECMO-VA.
ConclusãoOs doentes que colocaram ECMO após falência respiratória tiveram sobrevivência superior aos que colocaram após falência cardíaca. No ECMO-VA, a necessidade de mais fármacos inotrópicos foi preditor de mortalidade. Este é o primeiro registo publicado com a experiência global com ECMO num hospital terciário, em Portugal.
myocardial infarction
acute renal failure
acute respiratory distress syndrome
aspartate aminotransferase
cardiac care unit
extracorporeal membrane oxygenation
Extracorporeal Life Support Organization
intensive care unit
heart rate
invasive mechanical ventilation
left ventricle
renal replacement therapy
venoarterial extracorporeal membrane oxygenation
venovenous extracorporeal membrane oxygenation
Extracorporeal membrane oxygenation (ECMO) is a rescue therapy used to support patients with severe cardiac and/or pulmonary dysfunction refractory to conventional treatment. It was first used successfully in adults over 40 years ago, but its use has only recently become widespread, with technological advances in the available devices and with growing evidence of its effectiveness.1,2
The basic elements of an ECMO circuit are two cannulas (inflow and outflow), a centrifugal pump and an oxygenator. The latter is a gas exchanger containing a semipermeable membrane separating two chambers, one for blood and the other for gas. Deoxygenated blood is drained by the external pump, passes through the oxygenator, in which carbon dioxide is exchanged for oxygen, and is returned to the patient. When the blood is both drained from and returned to the venous system, the circuit is termed venovenous (VV) ECMO and only provides respiratory support; if it is drained from the venous system and returned via an artery, it is termed venoarterial (VA) ECMO and provides both respiratory and circulatory support.2
ECMO devices are light and portable, and so patients undergoing the treatment are relatively mobile, facilitating transport within and between hospitals, and the cannulas can be placed percutaneously or centrally, depending on clinical circumstances; the technique can be used in cases of cardiac arrest. Although ECMO provides only temporary support and has limitations, its versatility makes it useful in a variety of clinical situations, from clinical stabilization leading to complete recovery to situations requiring a bridge to decision, whether for long-term ventricular support or transplantation, or for suspension of support.3 The latest European Society of Cardiology guidelines on heart failure recommend VA-ECMO for patients in Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) levels 1 or 2.4
The aim of the present study is to describe patients’ demographic characteristics, indications, comorbidities, and physiological variables before ECMO; treatment (duration, modality, and complications); and mortality (on ECMO and in-hospital) of a population treated by ECMO in a single tertiary hospital. We also analyze possible predictors of mortality in this population.
MethodsThis was a retrospective observational cohort study that included all patients supported with ECMO in two different units – general intensive care (ICU) and cardiac care (CCU) – of a tertiary hospital, from the first patient cannulated in April 2011 up to October 2016. Patients in whom ECMO was used following cardiotomy were excluded from the analysis as they were treated according to different implantation and follow-up protocols.
During this period a total of 48 ECMO devices were placed in these units, 29 for cardiac failure and 19 for respiratory failure.
Relevant variables were selected on the basis of the published literature and patient data (demographic characteristics, comorbidities, and indications for ECMO) were collected from medical records. The values of these variables before ECMO considered for analysis were the worst in the first 24 h following admission to the unit. The Modification of Diet in Renal Disease (MDRD) formula was used to estimate glomerular filtration rate (GFR). ECMO-related complications and duration of ECMO support and of stay in the unit were recorded. Complications were classified as bleeding (access site, gastrointestinal or cerebral), ischemic, or access-related (thrombosis, dissection, aneurysm, pseudoaneurysm, or fistula). Acute renal failure (ARF) was defined according to the RIFLE classification. Hematological dysfunction was defined as platelets <100000/μl or hematocrit <35%, and liver dysfunction as transaminases more than three times the upper normal limit. Data were collected on mortality during and after ECMO and during hospital stay. Successful weaning was defined as that occurring with the intention to survive or as a bridge to another type of support. Comparisons were performed between the VA-ECMO and VV-ECMO groups and between survivors and non-survivors in each group. Survival after VA-ECMO was predicted by the SAVE-score, which uses a scale of -35 to 17 points based on diagnosis, age, weight, other acute or chronic dysfunction, ventilation parameters, duration of intubation before ECMO, pre-ECMO cardiac arrest, and hemodynamic parameters. The SAVE-score stratifies patients in five risk classes according to expected survival: class I (score >5) 75% survival; class II (1 to 5) 58%; class III (-4 to 0) 42%; class IV (-9 to -5) 30%; and class V (≤-10) 18%.5
All ECMO devices were placed percutaneously, either in the unit or in the catheterization laboratory, by a multidisciplinary team composed of intensivists, cardiologists, perfusionists and nurses. The Cardiohelp system (Maquet, Hirrlingen, Germany) was used in all cases except one. Femoral access was used in most cases of VA-ECMO and one femoral and one jugular access for VV-ECMO. A distal reperfusion cannula was used routinely when the indication for ECMO was myocardial infarction (MI), and for other indications when there was clinical evidence of poor distal perfusion, under echographic guidance in all cases. Anticoagulation was achieved by a bolus of unfractionated heparin at the time of cannulation (50-100 U/kg, maximum 5000 U), followed by perfusion adjusted to maintain a target activated partial thromboplastin time of 1.5 times the upper normal limit.
Surgical ventricular assistance was used when pharmacological and mechanical attempts to maintain adequate left ventricular venting were unsuccessful. Patients were referred for cardiac transplantation (not available in our center) when their etiology or clinical course indicated that left ventricular function was unlikely to recover. We recently established a referral protocol with one of the largest national transplantation centers.
Statistical analysisThe statistical analysis was performed using IBM SPSS version 19. Continuous variables are presented as mean and standard deviation and compared with the Student's t test or the Mann-Whitney U test, depending on normality. Categorical variables are presented as percentages and compared using the chi-square test or Fisher's test, as appropriate. All results are two-sided and a p value of <0.05 was considered statistically significant.
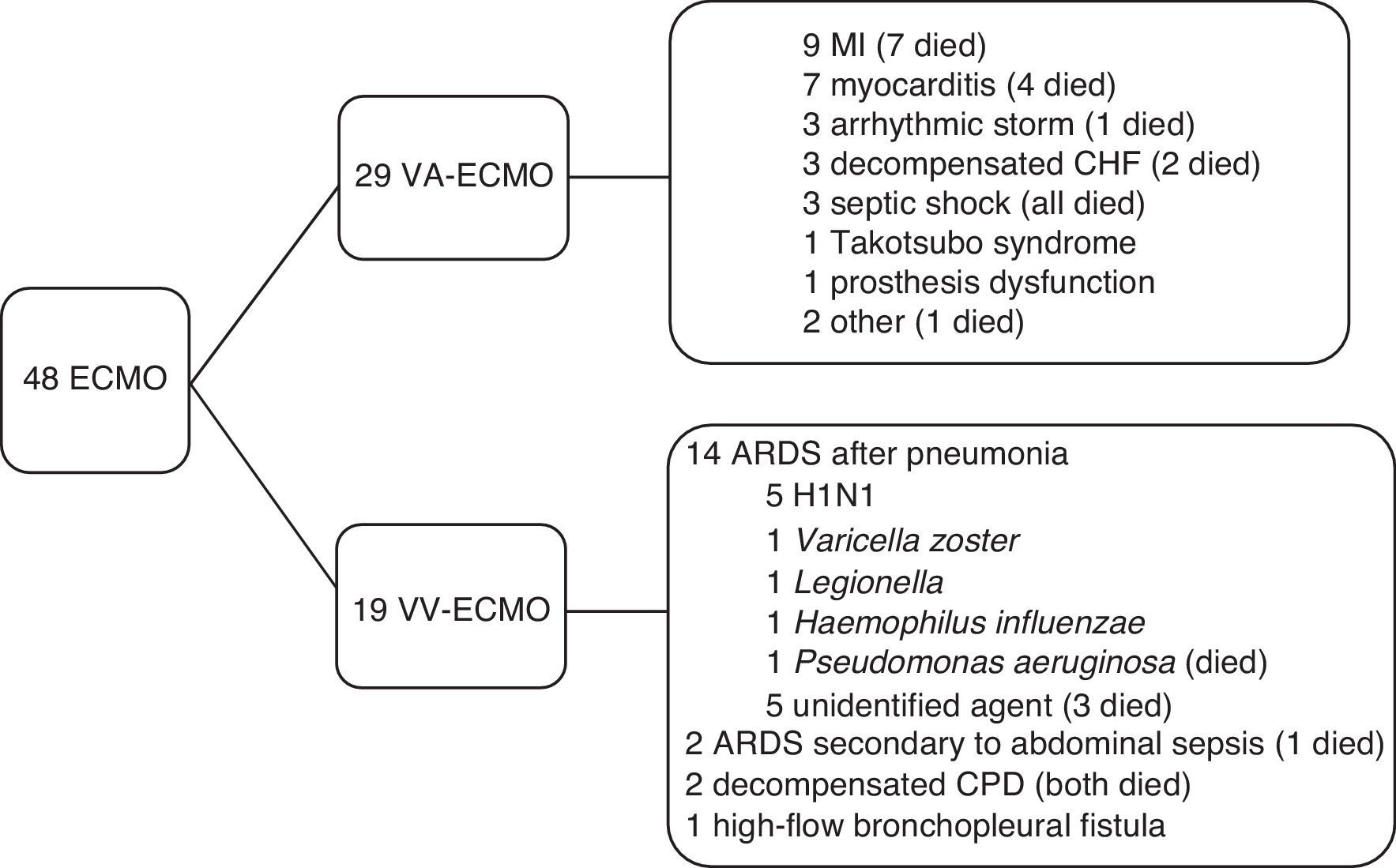
ResultsPopulation characteristicsBetween April 2011 and October 2016, 48 patients underwent ECMO: 29 VA-ECMO and 19 VV-ECMO. The most common indication for VA-ECMO was MI progressing to cardiogenic shock, while the most frequent indication for VV-ECMO was acute respiratory distress syndrome (ARDS) secondary to viral infection. These and other indications are shown in Figure 1.
Primary indications for placement of extracorporeal membrane oxygenation and mortality. ARDS: acute respiratory distress syndrome; CHF: chronic heart failure; CPD: chronic pulmonary disease; ECMO: extracorporeal membrane oxygenation; MI: myocardial infarction; VA-ECMO: venoarterial extracorporeal membrane oxygenation; VV-ECMO: venovenous extracorporeal membrane oxygenation.
Of the patients receiving VA-ECMO, 26 were admitted to the CCU and three to the ICU. The latter were suffering from cardiovascular failure secondary to severe respiratory infection.
Mean age in this group was 51.9±11.6 years and 51.7% were male. Around half had been transferred from another hospital in cardiogenic shock. Mean time between diagnosis and device placement was 51 h, and 12 patients (41%) were ventilated and cannulated at the same time. Thirteen suffered in-hospital cardiac arrest before ECMO placement, mean time for return of spontaneous circulation being 8.5 min; in three of these patients ECMO was placed during cardiac arrest. The mean SAVE-score was -4.2±4.9, median -4.5 (interquartile range [IQR] -0.25/-8). Left ventricular venting devices were used in eight patients (five intra-aortic balloon pump and three Impella CP® heart pump). One patient required a second cannula in the jugular vein to optimize coronary and cerebral oxygenation (venoarterial-venous ECMO).
Venovenous extracorporeal membrane oxygenationOf the patients supported by VV-ECMO, 17 were admitted to the ICU and two to the CCU, the latter due to ARDS secondary to severe pneumonia. Mean age in this group was 48.5±13.1 years and 52.6% were male. Mean time between diagnosis and device placement was 9.5 days. At the time of ECMO placement, all patients had been ventilated, the mean time between beginning of mechanical ventilation and device placement being 4.6 days. Of the 16 patients treated by VV-ECMO for ARDS, prone positioning was used in 10 before ECMO, and neuromuscular blockade was used in all except one. All patients had a Murray score >3.
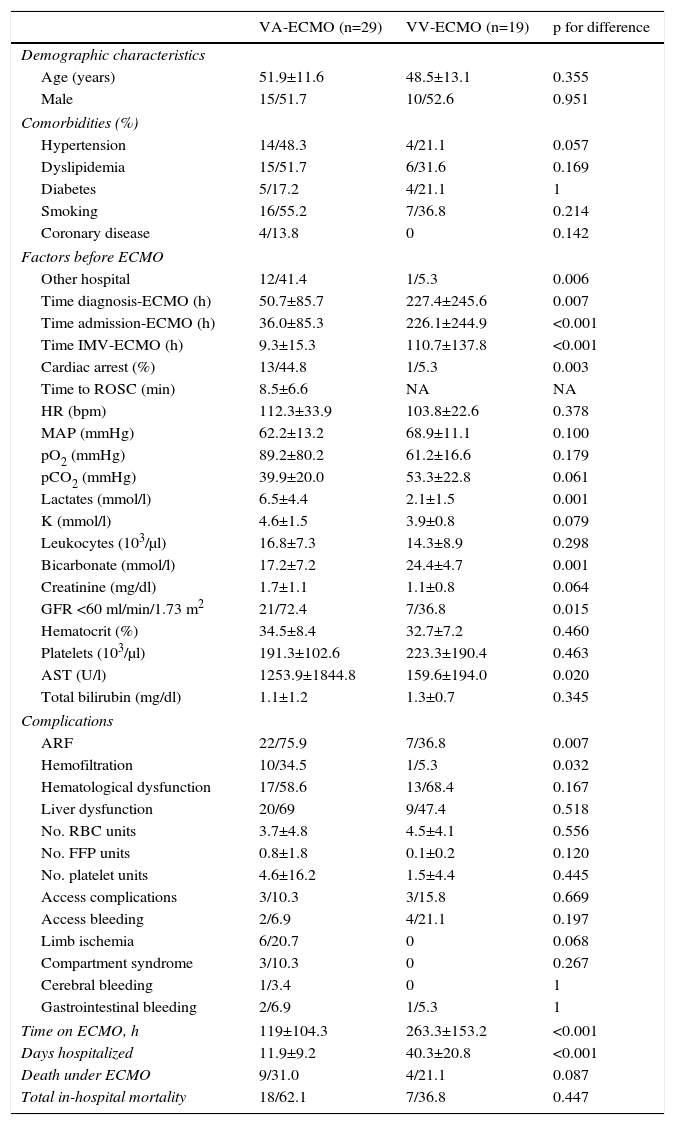
Venoarterial vs. venovenous extracorporeal membrane oxygenationComparison between the two ECMO modalities shows that VA-ECMO patients were more likely to have been transferred from another hospital (41.4 vs. 5.3%, p=0.006), had shorter time between diagnosis and device placement (50.7 vs. 227.4 h, p=0.007), and shorter previous invasive mechanical ventilation (IMV) time (9.3 vs. 110.7 h, p<0.001). Cardiac arrest before ECMO was more frequent in patients receiving VA-ECMO (44.8 vs. 5.3%, p=0.003). In the first 24 h of their stay in the unit, VA-ECMO patients had higher mean serum lactate (6.5 vs. 2.1 mmol/l, p=0.001) and aspartate aminotransferase (AST) levels (1253.9 vs. 159.6 U/l, p=0.02), more frequent moderate to severe renal failure (72.4% vs. 36.8%, p=0.015), and lower serum bicarbonate (17.2 vs. 24.4 mmol/l, p=0.001) than those receiving VV-ECMO.
Table 1 shows the characteristics of the study population.
Characteristics of the study population and comparison between patients receiving venoarterial and venovenous extracorporeal membrane oxygenation.
| VA-ECMO (n=29) | VV-ECMO (n=19) | p for difference | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 51.9±11.6 | 48.5±13.1 | 0.355 |
| Male | 15/51.7 | 10/52.6 | 0.951 |
| Comorbidities (%) | |||
| Hypertension | 14/48.3 | 4/21.1 | 0.057 |
| Dyslipidemia | 15/51.7 | 6/31.6 | 0.169 |
| Diabetes | 5/17.2 | 4/21.1 | 1 |
| Smoking | 16/55.2 | 7/36.8 | 0.214 |
| Coronary disease | 4/13.8 | 0 | 0.142 |
| Factors before ECMO | |||
| Other hospital | 12/41.4 | 1/5.3 | 0.006 |
| Time diagnosis-ECMO (h) | 50.7±85.7 | 227.4±245.6 | 0.007 |
| Time admission-ECMO (h) | 36.0±85.3 | 226.1±244.9 | <0.001 |
| Time IMV-ECMO (h) | 9.3±15.3 | 110.7±137.8 | <0.001 |
| Cardiac arrest (%) | 13/44.8 | 1/5.3 | 0.003 |
| Time to ROSC (min) | 8.5±6.6 | NA | NA |
| HR (bpm) | 112.3±33.9 | 103.8±22.6 | 0.378 |
| MAP (mmHg) | 62.2±13.2 | 68.9±11.1 | 0.100 |
| pO2 (mmHg) | 89.2±80.2 | 61.2±16.6 | 0.179 |
| pCO2 (mmHg) | 39.9±20.0 | 53.3±22.8 | 0.061 |
| Lactates (mmol/l) | 6.5±4.4 | 2.1±1.5 | 0.001 |
| K (mmol/l) | 4.6±1.5 | 3.9±0.8 | 0.079 |
| Leukocytes (103/μl) | 16.8±7.3 | 14.3±8.9 | 0.298 |
| Bicarbonate (mmol/l) | 17.2±7.2 | 24.4±4.7 | 0.001 |
| Creatinine (mg/dl) | 1.7±1.1 | 1.1±0.8 | 0.064 |
| GFR <60 ml/min/1.73 m2 | 21/72.4 | 7/36.8 | 0.015 |
| Hematocrit (%) | 34.5±8.4 | 32.7±7.2 | 0.460 |
| Platelets (103/μl) | 191.3±102.6 | 223.3±190.4 | 0.463 |
| AST (U/l) | 1253.9±1844.8 | 159.6±194.0 | 0.020 |
| Total bilirubin (mg/dl) | 1.1±1.2 | 1.3±0.7 | 0.345 |
| Complications | |||
| ARF | 22/75.9 | 7/36.8 | 0.007 |
| Hemofiltration | 10/34.5 | 1/5.3 | 0.032 |
| Hematological dysfunction | 17/58.6 | 13/68.4 | 0.167 |
| Liver dysfunction | 20/69 | 9/47.4 | 0.518 |
| No. RBC units | 3.7±4.8 | 4.5±4.1 | 0.556 |
| No. FFP units | 0.8±1.8 | 0.1±0.2 | 0.120 |
| No. platelet units | 4.6±16.2 | 1.5±4.4 | 0.445 |
| Access complications | 3/10.3 | 3/15.8 | 0.669 |
| Access bleeding | 2/6.9 | 4/21.1 | 0.197 |
| Limb ischemia | 6/20.7 | 0 | 0.068 |
| Compartment syndrome | 3/10.3 | 0 | 0.267 |
| Cerebral bleeding | 1/3.4 | 0 | 1 |
| Gastrointestinal bleeding | 2/6.9 | 1/5.3 | 1 |
| Time on ECMO, h | 119±104.3 | 263.3±153.2 | <0.001 |
| Days hospitalized | 11.9±9.2 | 40.3±20.8 | <0.001 |
| Death under ECMO | 9/31.0 | 4/21.1 | 0.087 |
| Total in-hospital mortality | 18/62.1 | 7/36.8 | 0.447 |
Categorical variables are presented as number (n) and percentage (%) and continuous variables as mean±standard deviation.
ARF: acute renal failure; AST: aspartate aminotransferase; ECMO: extracorporeal membrane oxygenation; FFP: fresh frozen plasma; GFR: glomerular filtration rate; HR: heart rate; IVM: invasive mechanical ventilation; K: potassium; MAP: mean arterial pressure; NA: not applicable; pCO2: partial pressure of carbon dioxide; pO2: partial pressure of oxygen; RBC: red blood cell; ROSC: return of spontaneous circulation.
The most frequent complication in the VA-ECMO group was lower limb ischemia, with a prevalence of 20.7% (six patients). Three of these required urgent fasciotomy for compartment syndrome, and one underwent below-knee amputation due to irreversible ischemia.
In VV-ECMO patients, the most common complications were access-related: 21.1% access site bleeding and 15.8% vessel-related complications (one patient with thrombus detected in the cannula during removal, one with probable superior vena cava syndrome and one with a low-flow fistula between the femoral vein and artery). In this group there was only one major bleeding complication, upper digestive tract bleeding due to a complicated stress-induced gastric ulcer that required surgical intervention.
Although bleeding complications were more frequent in the VV-ECMO group, there was no statistically significant difference between the groups in the need for transfusion of blood products. There was one case of heparin-induced thrombocytopenia, resolved with lepirudin, and one case of disseminated intravascular coagulation resulting in death. ARF with need for renal replacement therapy (RRT) was significantly more frequent in patients receiving VA-ECMO (ARF: 75.9% vs. 36.8%, p=0.007 and RRT: 34.5% vs. 5.3%, p=0.032).
Hematological dysfunction was more common in the VV-ECMO group (68.4% of patients).
Destination and mortalityECMO was used as a bridge to transplantation or to a long-term ventricular support device in 12 patients. Nine patients receiving VA-ECMO were referred for transplantation, of whom three were transplanted (only one survived), two received a Berlin Heart assist device but died, and the others died awaiting transplantation. Of the VV-ECMO group, three were referred for lung transplantation, of whom two died while on the waiting list and one was refused and support was withdrawn. The two patients who died awaiting transplantation were breathing spontaneously and not sedated, on an active rehabilitation program in the ICU.
Successful weaning took place in 69% of the VA-ECMO patients and in 78.9% of those receiving VV-ECMO. Mean ECMO time was significantly shorter in the VA-ECMO group (119.0±104.3 vs. 263.3±153.2 h, p<0.001), and hospitalization time was also significantly shorter (11.9±9.2 vs. 40.3±20.8 days, p<0.001), than in the VV-ECMO group.
Survival to discharge was 37.9% for VA-ECMO and 63.2% for VV-ECMO. It should be noted that survival in both groups was affected by the inclusion of patients awaiting organ transplantation.
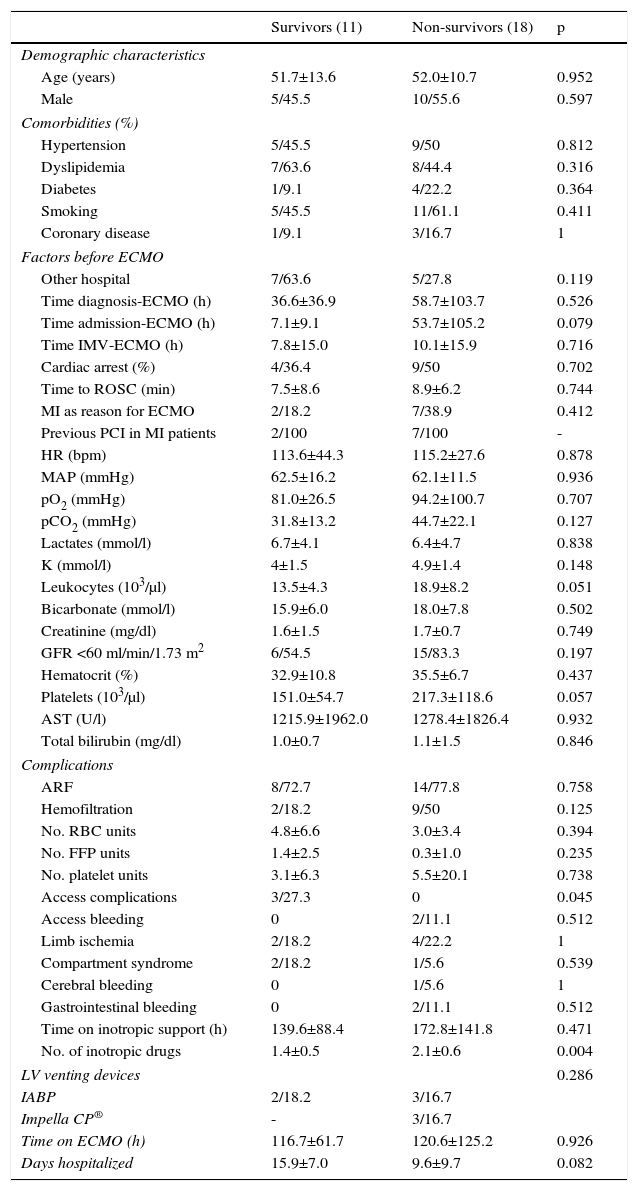
Predictors of mortalityOn univariate analysis, the only predictor of mortality in the VA-ECMO group was the mean number of inotropic drugs used (1.4±0.5 vs. 2.1±0.6, p=0.004). There was also a trend for lower leukocyte levels at admission (13.5±4.3 vs. 18.9±8.2×103/μl, p=0.051) and longer hospital stay (15.9±7.0 vs. 9.6±9.7 days, p=0.082) in survivors.
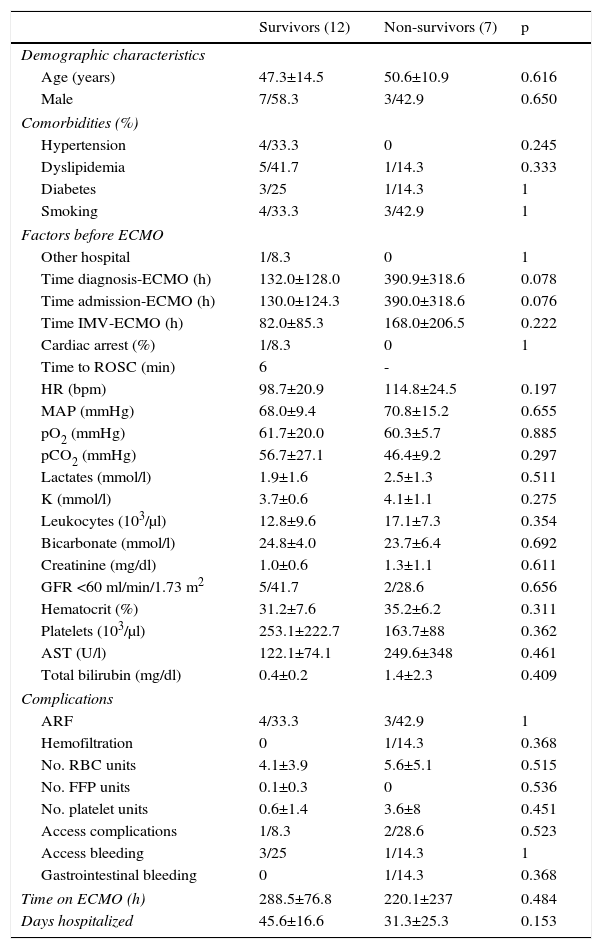
Survivors in the VV-ECMO group tended to have a longer time between diagnosis and device placement (132.0±128.0 vs. 390.9±318.6 h, p=0.078) (Tables 2 and 3).
Comparison between survivors and non-survivors among patients receiving venoarterial extracorporeal membrane oxygenation.
| Survivors (11) | Non-survivors (18) | p | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 51.7±13.6 | 52.0±10.7 | 0.952 |
| Male | 5/45.5 | 10/55.6 | 0.597 |
| Comorbidities (%) | |||
| Hypertension | 5/45.5 | 9/50 | 0.812 |
| Dyslipidemia | 7/63.6 | 8/44.4 | 0.316 |
| Diabetes | 1/9.1 | 4/22.2 | 0.364 |
| Smoking | 5/45.5 | 11/61.1 | 0.411 |
| Coronary disease | 1/9.1 | 3/16.7 | 1 |
| Factors before ECMO | |||
| Other hospital | 7/63.6 | 5/27.8 | 0.119 |
| Time diagnosis-ECMO (h) | 36.6±36.9 | 58.7±103.7 | 0.526 |
| Time admission-ECMO (h) | 7.1±9.1 | 53.7±105.2 | 0.079 |
| Time IMV-ECMO (h) | 7.8±15.0 | 10.1±15.9 | 0.716 |
| Cardiac arrest (%) | 4/36.4 | 9/50 | 0.702 |
| Time to ROSC (min) | 7.5±8.6 | 8.9±6.2 | 0.744 |
| MI as reason for ECMO | 2/18.2 | 7/38.9 | 0.412 |
| Previous PCI in MI patients | 2/100 | 7/100 | - |
| HR (bpm) | 113.6±44.3 | 115.2±27.6 | 0.878 |
| MAP (mmHg) | 62.5±16.2 | 62.1±11.5 | 0.936 |
| pO2 (mmHg) | 81.0±26.5 | 94.2±100.7 | 0.707 |
| pCO2 (mmHg) | 31.8±13.2 | 44.7±22.1 | 0.127 |
| Lactates (mmol/l) | 6.7±4.1 | 6.4±4.7 | 0.838 |
| K (mmol/l) | 4±1.5 | 4.9±1.4 | 0.148 |
| Leukocytes (103/μl) | 13.5±4.3 | 18.9±8.2 | 0.051 |
| Bicarbonate (mmol/l) | 15.9±6.0 | 18.0±7.8 | 0.502 |
| Creatinine (mg/dl) | 1.6±1.5 | 1.7±0.7 | 0.749 |
| GFR <60 ml/min/1.73 m2 | 6/54.5 | 15/83.3 | 0.197 |
| Hematocrit (%) | 32.9±10.8 | 35.5±6.7 | 0.437 |
| Platelets (103/μl) | 151.0±54.7 | 217.3±118.6 | 0.057 |
| AST (U/l) | 1215.9±1962.0 | 1278.4±1826.4 | 0.932 |
| Total bilirubin (mg/dl) | 1.0±0.7 | 1.1±1.5 | 0.846 |
| Complications | |||
| ARF | 8/72.7 | 14/77.8 | 0.758 |
| Hemofiltration | 2/18.2 | 9/50 | 0.125 |
| No. RBC units | 4.8±6.6 | 3.0±3.4 | 0.394 |
| No. FFP units | 1.4±2.5 | 0.3±1.0 | 0.235 |
| No. platelet units | 3.1±6.3 | 5.5±20.1 | 0.738 |
| Access complications | 3/27.3 | 0 | 0.045 |
| Access bleeding | 0 | 2/11.1 | 0.512 |
| Limb ischemia | 2/18.2 | 4/22.2 | 1 |
| Compartment syndrome | 2/18.2 | 1/5.6 | 0.539 |
| Cerebral bleeding | 0 | 1/5.6 | 1 |
| Gastrointestinal bleeding | 0 | 2/11.1 | 0.512 |
| Time on inotropic support (h) | 139.6±88.4 | 172.8±141.8 | 0.471 |
| No. of inotropic drugs | 1.4±0.5 | 2.1±0.6 | 0.004 |
| LV venting devices | 0.286 | ||
| IABP | 2/18.2 | 3/16.7 | |
| Impella CP® | - | 3/16.7 | |
| Time on ECMO (h) | 116.7±61.7 | 120.6±125.2 | 0.926 |
| Days hospitalized | 15.9±7.0 | 9.6±9.7 | 0.082 |
Categorical variables are presented as number (n) and percentage (%) and continuous variables as mean±standard deviation.
ARF: acute renal failure; AST: aspartate aminotransferase; ECMO: extracorporeal membrane oxygenation; FFP: fresh frozen plasma; GFR: glomerular filtration rate; HR: heart rate; IABP: intra-aortic balloon pump; IVM: invasive mechanical ventilation; K: potassium; LV: left ventricular; MAP: mean arterial pressure; MI: myocardial infarction; NA: not applicable; PCI: percutaneous coronary intervention; pCO2: partial pressure of carbon dioxide; pO2: partial pressure of oxygen; RBC: red blood cell; ROSC: return of spontaneous circulation.
Comparison between survivors and non-survivors among patients receiving venovenous extracorporeal membrane oxygenation.
| Survivors (12) | Non-survivors (7) | p | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 47.3±14.5 | 50.6±10.9 | 0.616 |
| Male | 7/58.3 | 3/42.9 | 0.650 |
| Comorbidities (%) | |||
| Hypertension | 4/33.3 | 0 | 0.245 |
| Dyslipidemia | 5/41.7 | 1/14.3 | 0.333 |
| Diabetes | 3/25 | 1/14.3 | 1 |
| Smoking | 4/33.3 | 3/42.9 | 1 |
| Factors before ECMO | |||
| Other hospital | 1/8.3 | 0 | 1 |
| Time diagnosis-ECMO (h) | 132.0±128.0 | 390.9±318.6 | 0.078 |
| Time admission-ECMO (h) | 130.0±124.3 | 390.0±318.6 | 0.076 |
| Time IMV-ECMO (h) | 82.0±85.3 | 168.0±206.5 | 0.222 |
| Cardiac arrest (%) | 1/8.3 | 0 | 1 |
| Time to ROSC (min) | 6 | - | |
| HR (bpm) | 98.7±20.9 | 114.8±24.5 | 0.197 |
| MAP (mmHg) | 68.0±9.4 | 70.8±15.2 | 0.655 |
| pO2 (mmHg) | 61.7±20.0 | 60.3±5.7 | 0.885 |
| pCO2 (mmHg) | 56.7±27.1 | 46.4±9.2 | 0.297 |
| Lactates (mmol/l) | 1.9±1.6 | 2.5±1.3 | 0.511 |
| K (mmol/l) | 3.7±0.6 | 4.1±1.1 | 0.275 |
| Leukocytes (103/μl) | 12.8±9.6 | 17.1±7.3 | 0.354 |
| Bicarbonate (mmol/l) | 24.8±4.0 | 23.7±6.4 | 0.692 |
| Creatinine (mg/dl) | 1.0±0.6 | 1.3±1.1 | 0.611 |
| GFR <60 ml/min/1.73 m2 | 5/41.7 | 2/28.6 | 0.656 |
| Hematocrit (%) | 31.2±7.6 | 35.2±6.2 | 0.311 |
| Platelets (103/μl) | 253.1±222.7 | 163.7±88 | 0.362 |
| AST (U/l) | 122.1±74.1 | 249.6±348 | 0.461 |
| Total bilirubin (mg/dl) | 0.4±0.2 | 1.4±2.3 | 0.409 |
| Complications | |||
| ARF | 4/33.3 | 3/42.9 | 1 |
| Hemofiltration | 0 | 1/14.3 | 0.368 |
| No. RBC units | 4.1±3.9 | 5.6±5.1 | 0.515 |
| No. FFP units | 0.1±0.3 | 0 | 0.536 |
| No. platelet units | 0.6±1.4 | 3.6±8 | 0.451 |
| Access complications | 1/8.3 | 2/28.6 | 0.523 |
| Access bleeding | 3/25 | 1/14.3 | 1 |
| Gastrointestinal bleeding | 0 | 1/14.3 | 0.368 |
| Time on ECMO (h) | 288.5±76.8 | 220.1±237 | 0.484 |
| Days hospitalized | 45.6±16.6 | 31.3±25.3 | 0.153 |
Categorical variables are presented as number (n) and percentage (%) and continuous variables as mean±standard deviation.
ARF: acute renal failure; AST: aspartate aminotransferase; ECMO: extracorporeal membrane oxygenation; FFP: fresh frozen plasma; GFR: glomerular filtration rate; HR: heart rate; IVM: invasive mechanical ventilation; K: potassium; MAP: mean arterial pressure; MI: myocardial infarction; NA: not applicable; PCI: percutaneous coronary intervention; pCO2: partial pressure of carbon dioxide; pO2: partial pressure of oxygen; RBC: red blood cell; ROSC: return of spontaneous circulation.
Over a five-year period, 48 patients underwent ECMO: 29 for cardiovascular failure and 19 for respiratory failure. Table 1 shows that disease markers, at least initially, in patients receiving VA-ECMO were more severe, with higher serum lactate and AST levels, greater prevalence of moderate to severe renal dysfunction, and lower serum bicarbonate. The shorter time between diagnosis and ECMO placement and the greater frequency of previous IMV in this group are explained by the greater severity of clinical presentation. Almost half of the VA-ECMO group were transferred in cardiogenic shock from another hospital, and were thus more likely to have been suffering shock for longer and to have more advanced organ dysfunction.
Percutaneous left ventricular decompression techniques were used in around 30% of patients in our cohort. Other measures, including reduction of afterload, use of arterial vasodilators and inotropic drugs, fluid restriction and hemofiltration can help prevent ventricular distension, and were applied systematically in all cases.6–8 This complication, which occurs in 10-60% of patients undergoing VA-ECMO and is more frequent in newborns and children,7 hampers recovery and leads to pulmonary edema.
Anticoagulation is a challenge in these patients, as contact between the blood and the extracorporeal circuit leads to activation and consumption of pro- and anticoagulant factors, meaning a delicate balance must be struck between risk of thrombosis and of bleeding.9 The recommended anticoagulant is unfractionated heparin.9,10 All patients in our population underwent continuous heparin infusion and only one was diagnosed with heparin-induced thrombocytopenia, which necessitated replacing heparin with lepirudin.
Although ECMO is increasingly used to support patients in cardiac or respiratory failure, it is often associated with complications, some of which can have a significant impact on the quality of life of survivors.6 Bleeding complications occurred in 10 patients (21%) in our series. The most frequent bleeding site was the point of insertion of the cannulas. In our cohort, bleeding and quantity of transfused blood products were not predictors of mortality, although this has been reported in the literature.3,11–13 This is probably due to the small sample size, which meant these parameters did not reach statistical significance. Neurological complications, the incidence of which in the literature ranges between 15% and 37%,14,15 were rare in our series, in only one patient (spontaneous intracerebral bleeding). This low figure is probably due to underuse of cerebral computed tomography and the lack of postmortem anatomopathological studies.
Acute lower limb ischemia occurred only in the VA-ECMO group (6/29 patients). Use of a distal reperfusion cannula and contralateral venous cannulation are essential to reduce the incidence of this complication.16,17 Three patients required urgent fasciotomy (10.3% of the VA-ECMO group) and one underwent lower limb amputation. These results are similar to those in the literature, in which lower limb ischemia affects 10-30% of patients and compartment syndrome affects 8-14%.8,12,16,18–20
As in a meta-analysis by Cheng et al.,20 ARF was the complication most frequently associated with ECMO, affecting over 75% of those undergoing VA-ECMO and nearly 40% in those receiving VV-ECMO. Pump speed and red cell distribution width have recently been identified as predictors of ARF.21 In our population, 10 patients undergoing VA-ECMO and one receiving VV-ECMO required RRT. Although need for RRT reflects inadequate renal perfusion and/or direct injury to the kidneys by the support technique through hemolysis, transfusions, or rhabdomyolysis, it was not significantly associated with greater mortality in either of our groups. This may be explained by the small sample size, since several series have demonstrated that ARF and RRT are independent predictors of mortality in individuals receiving ECMO.6,11,19,22,23 In-hospital mortality was 36.8% in the VV-ECMO group and 62.1% in the VA-ECMO group. These figures are consistent with those observed in the international ELSO registry, in which in-hospital mortality in adults was 42% in VV-ECMO and 59% in VA-ECMO.24 In our series, 69% of patients undergoing VA-ECMO and 78.9% of those receiving VV-ECMO were successfully weaned.
CESAR was the first randomized trial to show clear benefits of VV-ECMO. This study randomized 180 patients with severe respiratory failure to a conservative strategy or to transfer to a center with ECMO, 63% of whom survived, as opposed to 47% of controls.25 The H1N1 pandemic in 2009 led to greater experience with ECMO in many centers,26 and in our center, five patients with ARDS secondary to H1N1 infection all survived.
In this study, univariate analysis revealed no predictors of mortality in VV-ECMO, whereas in published series previous pulmonary disease, advanced age, previous barotrauma and number of days on IMV before ECMO were all independent predictors of mortality.13,27,28
In VA-ECMO, a greater number of inotropic drugs used during hospitalization predicted death. In other series advanced age, female gender, high body mass index, diabetes, elevated serum lactate, number of red blood cell units transfused, and placement of ECMO during cardiac arrest were all independent predictors of mortality.3,6,18,23,29,32
Mortality in our sample was 77%. Analysis of mortality in terms of reasons for device placement shows that cardiogenic shock following MI and shock following cardiotomy has the highest mortality, most series reporting figures of 40-75%.18,29–32 In these series, ECMO placement in such situations occurred mainly before or during percutaneous revascularization, while in our population it was only placed as ventricular support when a patient remained in refractory cardiogenic shock after a successful percutaneous intervention, probably due to pump failure and thus probably with less likelihood of recovery.
In view of the high cost and high short-term mortality of ECMO, careful patient selection is vital. In 2015 Schmidt et al. published the SAVE-score, which is designed to predict survival after VA-ECMO.5 In our population, the mean SAVE-score was -4.2±4.9, median -4.5 (IQR -0.25/-8). Thus most of our patients were in risk class III or IV, with estimated survival of 30-42%, and actual survival was 37.9%. This score still needs to be validated but could be a useful tool in the future.5
LimitationsThe limitations of our study include its design as a retrospective cohort study without a control group. The sample size was small, and certain variables which the authors consider important could not be included due to gaps in the recording of certain data. There may therefore have been biases that could have influenced the results presented. In addition, this is a single-center analysis and hence generalization of the results is limited.
Despite these limitations, the study provides real-world results of the use of ECMO in a tertiary hospital.
ConclusionPatients with cardiovascular failure placed on VA-ECMO have much higher mortality than those with respiratory failure placed on VV-ECMO. In VV-ECMO, later device placement appears to be associated with a worse prognosis.
Complications were relatively common, particularly vascular complications. Given the frequency of hematological dysfunction and the difficulty of achieving proper hemostasis, ways of minimizing the consequences of vascular complications are a priority in the management of these patients.
The need for more inotropic drugs was a predictor of mortality in VA-ECMO.
In conclusion, despite the small sample size, our results are similar to those in most published series. This is the first published record of the overall experience with ECMO in a Portuguese tertiary hospital.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Passos Silva M, Caeiro D, Fernandes P, Guerreiro C, Vilela E, Ponte M, et al. Oxigenação por membrana extracorporal na falência circulatória e respiratória – experiência de um centro. Rev Port Cardiol. 2017;36:833–842.


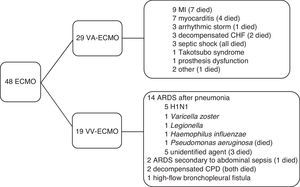 ARDS: acute respiratory distress syndrome; CHF: chronic heart failure; CPD: chronic pulmonary disease;
ARDS: acute respiratory distress syndrome; CHF: chronic heart failure; CPD: chronic pulmonary disease; 

