The major limitation of long-term survival after cardiac transplantation is allograft vasculopathy, which consists of concentric and diffuse intimal hyperplasia. The disease still has a significant incidence, estimated at 30% five years after cardiac transplantation. It is a clinically silent disease and so diagnosis is a challenge. Coronary angiography supplemented by intravascular ultrasound is the most sensitive diagnostic method. However, new non-invasive diagnostic techniques are likely to be clinically relevant in the future. The earliest possible diagnosis is essential to prevent progression of the disease and to improve its prognosis. A new nomenclature for allograft vasculopathy has been published in July 2010, developed by the International Society for Heart and Lung Transplantation (ISHLT), establishing a standardized definition. Simultaneously, the ISHLT published new guidelines standardizing the diagnosis and management of cardiac transplant patients. This paper reviews contemporary concepts in the pathophysiology, diagnosis, prevention and treatment of allograft vasculopathy, highlighting areas that are the subject of ongoing research.
A principal limitação da sobrevida a longo prazo após-transplante cardíaco é a doença vascular do aloenxerto que consiste na hiperplasia concêntrica e difusa da íntima arterial. A doença continua a ter uma incidência significativa estimada em 30% aos 5 anos pós-transplante cardíaco. Por ser uma doença clinicamente silenciosa, o seu diagnóstico é um desafio. A angiografia coronária complementada pela ecografia intravascular é o método de diagnóstico mais sensível. No entanto, novas técnicas de diagnóstico não invasivas podem vir a ter relevância clínica no futuro. O seu diagnóstico, o mais precocemente possível, é essencial de forma a permitir atrasar a progressão da doença a fim de melhorar o seu prognóstico. Em Julho de 2010, foi publicada uma nova nomenclatura recomendada para a vasculopatia do aloenxerto, elaborada pela Internacional Society for Heart and Lung Transplantation (ISHLT) e que permite uma uniformização da definição. Em simultâneo, foram publicadas as novas recomendações da ISHLT que procuram uma uniformização no diagnóstico e no manejo destes doentes. Este artigo faz uma revisão dos conceitos atuais da fisiopatologia, diagnóstico, prevenção e tratamento da vasculopatia do aloenxerto, realçando áreas em investigação.
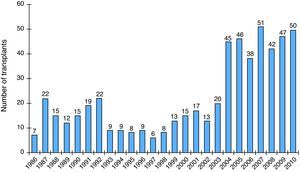
Despite the availability of a wide range of drug therapies and electrophysiological interventions such as resynchronization devices, cardiac transplantation is still the gold standard treatment for advanced heart failure refractory to medical therapy. Between 1983 and 2010, 89000 heart transplants were reported to the Registry of the International Society for Heart and Lung Transplantation (ISHLT), which estimates that the number of heart transplants being performed worldwide likely exceeds 5000 per year.1 The first cardiac transplant in Portugal was performed in 1986.2–4 Data from the Portuguese Authority for Blood and Transplantation Services show that the number of transplants in Portugal has increased in recent years, with a total of 558 patients up to 2010 (Figure 1).5 Mean survival after transplantation increased from 8.3 years in the 1980s to 10.4 years in the 1990s and is currently 13 years. This improvement reflects lower early mortality after transplantation, which is due to various factors including improved selection recipient and donor criteria, better preservation of donor hearts, and the introduction of cyclosporin in the early 1980s, which revolutionized immunosuppressive therapy and sharply reduced acute allograft rejection.6 However, long-term mortality (>1 year after transplantation) has remained fairly constant at 3–4% per year, higher than for the general population.1
One of the main factors limiting long-term survival after transplantation is cardiac allograft vasculopathy (CAV).7 The incidence of this condition, an accelerated form of coronary artery disease characterized by progressive thickening of the arterial intima, is significant (8% at one year, 20% at three years, 30% at five years, and more than 50% at 10 years), and it accounts for major morbidity and mortality.1 A consensus document was published in July 2010 by the ISHLT aiming to standardize the nomenclature of CAV.7 Simultaneously, the ISHLT (Task Force 3) published new guidelines for the care of heart transplant recipients, which include management of CAV.8 The aim of both documents is to improve early diagnosis of CAV so that appropriate treatment and prevention measures can be implemented. This paper reviews contemporary concepts in the pathophysiology, diagnosis, prevention and treatment of allograft vasculopathy, highlighting areas that are the subject of ongoing research.
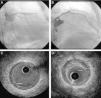
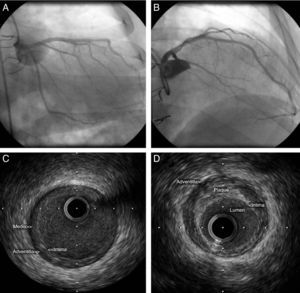
PathophysiologyThe pathological characteristics of CAV differ significantly from those of typical atherosclerotic coronary disease (Table 1). CAV consists of concentric and diffuse proliferation of the arterial intima, resulting in thickening and pathological remodeling that lead to progressive narrowing of the lumen,9 preferentially of small and medium-sized arteries.10,11 Veins and intramyocardial vessels are frequently involved. Calcium deposits are uncommon; the internal elastic lamina remains intact, while there may be inflammation with thickening of the intima by infiltration of mononuclear inflammatory cells.12 Eccentric plaques from the donor may also be detected very soon after transplantation, while at a later stage typical atherosclerotic plaques can be seen combined with the diffuse intimal thickening caused by CAV.13,14Figure 2 shows intravascular ultrasound (IVUS) images of two types of plaque in a patient transplanted five years previously and with apparently normal coronary angiography.
Differences between cardiac allograft vasculopathy and atherosclerotic coronary disease.
| Cardiac allograft vasculopathy | Atherosclerotic coronary disease | |
| Location | Distal epicardial and intramyocardial vessels | Proximal epicardial vessels |
| Plaque type | Diffuse, concentric | Focal, eccentric |
| Inflammation | Yes | Rarely |
| Vasculitis | Infrequently | Never |
| Internal elastic lamina | Intact | Disrupted |
| Calcium deposits | No | Yes |
Intravascular ultrasound images of the anterior descending artery in a patient transplanted five years previously, showing both the concentric intimal thickening typical of cardiac allograft vasculopathy (C) and an eccentric plaque typical of atherosclerotic coronary disease (D). Coronary angiography did not suggest disease in this artery (A and B).
CAV is a complex disease with a variety of etiologies that include immunological and non-immunological factors.15 Accelerated CAV results from a continuous local and systemic inflammatory response, with consequent repetitive vascular endothelial injury, triggered by alloantigen-dependent and non-immunological stress factors.16,17 A study by Raichlin et al.16 showed that the presence of “inflammatory” plaque (necrotic core and spotty dense calcification) in coronary arteries detected by virtual histology intravascular ultrasound was associated with early recurrent rejection and with subsequent progression to CAV, supporting the idea that the inflammatory process has an immunological basis. The study also highlighted the importance of local inflammation in the process of endothelial dysfunction. Several other studies have shown that systemic inflammation also contributes to this form of accelerated vasculopathy. High-sensitivity C-reactive protein, one of the most sensitive markers of systemic inflammation, is often elevated in patients who develop CAV and predicts late rejection. 17–21. Some authors suggest that endothelial dysfunction may represent an early and potentially reversible stage of CAV22,23; disruption of endothelial nitric oxide production and increased endothelin may promote atherogenesis by inducing vasoconstriction.24,25
Concerning possible immunological factors, endothelial cells express class I and II human leukocyte antigens (HLAs) of the major histocompatibility complex, which are targets of humoral and cell-mediated immune responses.15,26 Activated T lymphocytes secrete cytokines (interleukins, interferon and tumor necrosis factor) that recruit activated monocytes and macrophages and stimulate production of adhesion molecules in the endothelium.27 The macrophages produce cytokines and growth factors, leading to smooth muscle cell proliferation and extracellular matrix synthesis.28 Circulating anti-HLA antibodies and antibodies to donor endothelium are found in a significant number of transplant patients and are a sign of worse prognosis, supporting the idea that humoral immune responses also have a role in the pathogenesis of CAV. 29–32
Non-immunological risk factors include donor and recipient age,1 the cause of brain death,33 ischemia–reperfusion injury,34 viral infection35,36 and metabolic disorders.37 A study published in 2006 with a three-year follow-up by IVUS showed that the presence of coronary atherosclerosis in the donor heart does not contribute to more rapid progression of intimal hyperplasia and does not appear to affect long-term survival.38 Conventional cardiovascular risk factors such as hypertension, dyslipidemia and diabetes are often more prevalent in cardiac transplant patients than in the general population, largely due to the immunosuppressive therapy required to avoid rejection.39 Dyslipidemia and insulin resistance are the non-immunological factors that contribute most to the development of CAV simply because they are so common (in 50–80% of transplant recipients).37,40
DiagnosisAngiographyDiagnosis of CAV remains a challenge. Since it is clinically silent due to denervation of the allograft, major cardiovascular events such as myocardial infarction, heart failure and sudden death may occur without previous angina.41 The fact that it is a vasculopathy, affecting vessels in a diffuse manner, limits the use of non-invasive methods for early diagnosis that rely on the detection of lesions obstructing coronary flow.42 Coronary angiography is used in many transplant centers to diagnose CAV. In a large study by Costanzo et al.43 of 5693 angiograms from different centers screening for CAV by conventional coronary angiography, coronary disease was present in 42% of patients five years after transplantation. Of those with severe CAV, 50% died or were retransplanted. The classification of CAV used in this study had prognostic value and was therefore incorporated into the standardized nomenclature published by the ISHLT (Table 2).7
Recommended nomenclature for cardiac allograft vasculopathy.7
| ISHLT CAV0 (not significant) | No detectable angiographic lesion |
| ISHLT CAV1 (mild) | Left main stenosis <50%, or primary vessel with maximum lesion of <70%, or any branch stenosis <70% without allograft dysfunction |
| ISHLT CAV2 (moderate) | Left main stenosis ≥50%, single primary vessel ≥70%, or isolated branch stenosis ≥70% in branches of two systems, without allograft dysfunction |
| ISHLT CAV3 (severe) | Left main stenosis ≥50%, or two or more primary vessels ≥70% stenosis, or isolated branch stenosis ≥70% in all three systems; or ISHLT CAV1 or CAV2 with allograft dysfunction (defined as left ventricular ejection fraction ≤45% usually in the presence of regional wall motion abnormalities) or evidence of significant restrictive physiology. |
Definitions:
(a) Primary vessel: denotes the proximal and middle third of the left anterior descending artery, the left circumflex, the ramus and the dominant or co-dominant right coronary artery with the posterior descending and posterolateral branches.
(b) Branch vessel: includes the distal third of the primary vessels, diagonals and obtuse marginal branches or any portion of a non-dominant right coronary artery.
(c) Restrictive cardiac allograft physiology: symptomatic heart failure with echocardiographic E to A velocity ratio >2, isovolumetric relaxation time <60ms, deceleration time <150ms, or restrictive hemodynamic values (right atrial pressure >12mmHg, pulmonary capillary wedge pressure >25mmHg, cardiac index <2l/min/m2).
Although annual angiography is the recommended screening method, its sensitivity for detecting coronary disease in transplanted hearts is low (positive predictive value of only 44% compared to IVUS).44,45 Conventional angiography does not assess the arterial wall and the vascular remodeling that results from CAV may hinder its diagnosis.46 IVUS overcomes these limitations and is considered the gold standard exam and the most sensitive for early detection of CAV.47 Maximal intimal thickening evaluation should be based on automated pullback in one or more epicardial vessels over a 40–50-mm segment.7 Intimal thickening of >0.5mm in the first year post-transplant is a risk marker for mortality and nonfatal major cardiovascular events and predicts the development of CAV within five years.41,48 IVUS can thus be used to assess the risk of developing the disease, determine prognosis and guide therapy, but this indication is not fully consensual.7 The current ISHLT guidelines8 state that conventional coronary angiography should be performed annually or biannually to assess the development of CAV. Patients free of CAV at 3–5 years after transplantation, especially those with renal insufficiency, may undergo less frequent invasive evaluation. IVUS in conjunction with coronary angiography at 4–6 weeks after transplantation is an option if there is suspicion of donor coronary disease (donor age >35 years), but is not performed in most centers, while at one year it can detect rapidly progressive CAV and provide prognostic information. Follow-up coronary angiography is recommended six months after percutaneous coronary intervention because of high restenosis rates in cardiac transplant recipients.8
Assessment of endothelial and microvascular functionCAV affects not only the epicardial circulation but also the microcirculation, and tests of microvascular function have been developed, including assessment of coronary flow reserve (CFR)49 and microvascular resistance50 by thermodilution. The PITA study49 showed that fractional flow reserve measured using a pressure wire linked to a transducer correlates with IVUS findings, suggesting that the diffuse alterations in the coronary tree found in CAV are reflected in functional alterations, and that measuring CFR by thermodilution is a relatively simple way to obtain information on the involvement of the microvascular compartment in patients with angiographically normal coronary arteries. Studies have shown a good correlation between microvascular disease and prognosis.51,52
Non-invasive diagnostic methodsIn recent years non-invasive diagnostic techniques, particularly myocardial perfusion scintigraphy (MPS), dobutamine stress echocardiography (DSE), and computed tomography (CT) and magnetic resonance imaging (MRI) angiography have increasingly been used for assessment of CAV. They are particularly useful in transplant recipients in whom invasive studies are not possible and for monitoring pediatric patients, in order to minimize use of invasive methods, although their application in this context is not fully established.8
A study published in 2000 suggested that MPS could be used as a screening method in cardiac transplant recipients due to its ability to exclude severe coronary lesions requiring revascularization.53 Wu et al. subsequently confirmed the high negative predictive value (96%) of dobutamine stress scintigraphy in excluding significant coronary disease.54 Although this exam has low sensitivity for early detection of CAV, some authors have found that it has prognostic value.55,56
Serial transthoracic echocardiography is recommended to detect possible progressive deterioration in left ventricular function or restrictive diastolic dysfunction resulting from silent myocardial ischemia.7
The first study to compare DSE with IVUS, in 109 cardiac transplant recipients, showed that DSE detected CAV with a sensitivity of 72% and identified patients with worse prognosis with a comparable predictive value to IVUS and angiography.57 Other authors have demonstrated the prognostic value of this modality.58,59 More recent studies have shown the value of new quantitative echocardiographic techniques such as tissue Doppler and assessment of systolic strain for early detection of CAV.60,61 The microcirculation can also be assessed through quantification of coronary flow reserve by contrast echocardiography.62–64 Reduced CFR is an early marker of CAV and is associated with the occurrence of major cardiovascular events.65
Studies have suggested that 64-slice CT angiography is superior to conventional coronary angiography in identifying non-obstructive vasculopathy.66 In comparison to IVUS, CT angiography has high specificity (92%) and reasonable negative predictive value (77%) for detecting CAV,67 and may in the future have a role in monitoring the disease. Schepis et al. compared dual-source CT with IVUS in 30 cardiac transplant recipients with a mean heart rate of 80±14bpm. Although the high heart rates often seen in these patients may limit the technical quality of this non-invasive exam, the study showed that it has good diagnostic accuracy for CAV (specificity 84% and negative predictive value 91%).68
MRI angiography also has the advantage of being non-invasive and not requiring nephrotoxic contrast agents or exposure to ionizing radiation, but its sensitivity is still too low to be used to screen for CAV.69
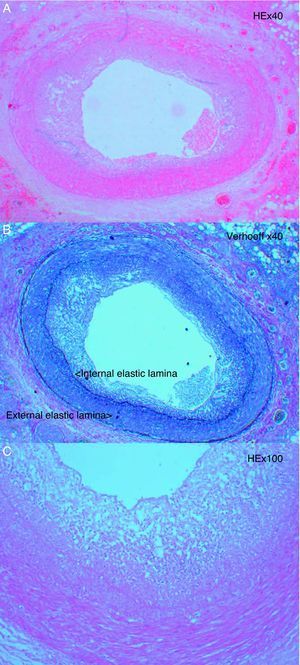
Endomyocardial biopsyEndomyocardial biopsy has limited sensitivity in identifying CAV, because the samples are from small intramyocardial arteries and arterioles in which the typical intimal proliferation (Figure 3) is not usually visible in the first years after transplantation.28
BiomarkersThere has been considerable research into immunological factors (including anti-vimentin70 and anti-HLA antibodies), genetics,71 and proteins (including C-reactive protein21, B-type natriuretic peptide,72 troponin I,73 and von Willebrand factor74), aiming to identify those that can be used as biomarkers of risk of CAV. However, none has yet been incorporated into clinical practice as a marker for defining severity of CAV, due to the lack of standardized methods for measurement, variability in sensitivity and specificity, and issues of reproducibility between laboratories.7
To summarize, non-invasive diagnostic exams, particularly CT angiography, can be used to exclude significant CAV but are not as sensitive as IVUS. DSE can be used as a prognostic tool. In the future, non-invasive imaging methods may replace coronary angiography for screening, with angiography in association with IVUS reserved for high-risk patients or those with inconclusive or positive results on non-invasive tests.7
PreventionTreatment for fully established CAV is limited due to the diffuse obliterative nature of the disease process. Preventive measures should therefore be begun as soon as possible, since intimal thickening usually occurs within a year of transplantation.75 Prevention before transplantation includes avoidance of endothelial damage by reducing ischemic time during donor organ harvest and transportation, while after transplantation primary prevention should include optimization of immunosuppressive therapy, aggressive control of traditional cardiovascular risk factors (hypertension, dyslipidemia, diabetes, obesity, smoking and sedentarism), and prophylaxis for cytomegalovirus (CMV) infection.8
Hypertension, dyslipidemia and diabetes are common in cardiac transplant recipients, frequently resulting from or aggravated by immunosuppressive therapy.1
The incidence of hypertension was 76% and 90% at one and five years, respectively.1 Cyclosporin therapy, through its direct effects and nephrotoxicity, is among the main factors increasing hypertension,76 which can also be triggered or aggravated by corticosteroids. There are no large randomized studies that demonstrate the superiority of a particular antihypertensive agent in this population. Since hypertension is typically difficult to control in these patients, it is often necessary to combine two or more antihypertensive drugs, normally a calcium channel blocker and an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.8 Weight loss, low sodium diet, and exercise are appropriate adjuncts to facilitate control of blood pressure in this population.8,76
The incidence of dyslipidemia was 74% and 91% at one and five years, respectively.1 Two randomized trials comparing pravastatin77 and simvastatin78 with placebo in cardiac transplant recipients showed that statins reduce the incidence of CAV and improve long-term prognosis. Other studies suggest that the beneficial effects of statins derive not only from cholesterol reduction but also from immunosuppressive effects.79 Statin therapy should accordingly be considered for all cardiac transplant patients, whatever their lipid profile.8
Diabetes affected 39% of transplant patients at five years.1 Calcineurin inhibitors, especially tacrolimus,80 and glucocorticoids81 contribute to this high prevalence, while other risk factors include pre-transplant glucose intolerance, family history of diabetes, and obesity.82 The ISHLT guidelines recommend avoidance of corticosteroid therapy if possible and low doses of calcineurin inhibitors. Patients should be periodically screened by measuring fasting plasma glucose levels or an oral glucose tolerance test and HbA1c determination, as appropriate; treatment of established diabetes in cardiac transplant recipients should be as in the general population.8
Patients should be encouraged to participate in cardiac rehabilitation programs, including both aerobic and resistance training, in order to modify cardiovascular risk factors.8,83,84
Antiviral therapy with ganciclovir or valganciclovir for existing CMV infection appears to slow progression of CAV,12 but the effects of CMV prophylaxis on prevention of CAV remain unclear.
Immediately after transplantation, patients usually begin triple immunosuppressive therapy with a calcineurin inhibitor (cyclosporine or tacrolimus) combined with azathioprine or mycophenolate mofetil, as well as corticosteroids.8 The evidence suggests that doses of calcineurin inhibitors should be reduced whenever possible, since they are associated with nephrotoxicity, adverse metabolic effects and endothelial dysfunction, and thus can contribute to the progression of CAV.85
Other drugs have been suggested as protective against progression of CAV, particularly the immunosuppressant mycophenolate mofetil which, rather than azathioprine, in combination with a calcineurin inhibitor results in less intimal thickening in the first year after transplantation, reflected in reduced mortality and retransplantation at 36 months.86
The antiproliferative properties of everolimus87 and sirolimus88 have been shown to reduce the severity and incidence of CAV at 12 and 24 months, respectively, compared to azathioprine. The ISHLT guidelines accordingly recommend that azathioprine or mycophenolate mofetil can be replaced by one of these drugs in cases of established CAV.8 Most centers do not use these agents in the acute post-transplant phase because their antiproliferative effects can delay healing of the surgical wound.
Appropriate management of immunosuppressive therapy is essential in the early stages of development of CAV, since some studies indicate that its progression can be slowed or even reversed.88,89
TreatmentIn cases of established CAV, percutaneous coronary intervention or coronary artery bypass grafting can be performed but these procedures are considered palliative since they do not alter disease progression, need for reintervention or overall survival.90–92 Coronary angioplasty is the treatment of choice for severe focal lesions, since success rates are high and complications are few, but the risk of restenosis is higher than in the general population.93 Restenosis rates are lower with drug-eluting stents than with bare-metal stents, although survival is similar with both types.93–96
The only definitive therapy for CAV is retransplantation, which may be considered for patients with advanced CAV without contraindications.97 Although overall survival after retransplantation is lower than for primary transplantation, when it is performed more than five years after the original transplant, one-year survival is comparable to the primary transplant.1 Retransplantation for CAV is also associated with better survival than for other causes of retransplantation.98
ConclusionThe etiology of CAV is multifactorial. It is the major limitation to long-term survival after cardiac transplantation. Early diagnosis is a challenge but important as it enables the disease to be treated in the initial stages, preventing its progression and improving prognosis. Coronary angiography remains the recommended method to diagnose CAV; its sensitivity is increased when supplemented by IVUS. New non-invasive techniques are likely to be clinically relevant in the future. Standardization of treatment between centers is essential to improve management of cardiac transplant recipients, since the only way to improve overall survival is through combined efforts for earlier detection, better prevention and more aggressive treatment of CAV.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Dr. Sância Ramos of the Pathology Laboratory of Hospital de Santa Cruz for her assistance with the study of the pathology of CAV.
Please cite this article as: Calé R, et al. Diagnóstico, prevenção e tratamento da doença vascular do aloenxerto. Rev Port Cardiol. 2012. doi:10.1016/j.repc.2012.08.001.