Cardioembolism is one of the most common causes of ischemic stroke, with an estimated prevalence of 20–30%, and correct diagnosis is essential given the therapeutic implications. Although stroke risk scores (CHADS2 and more recently CHA2DS2-VASc) have been validated in heterogeneous populations of patients with atrial fibrillation, their accuracy has not been ascertained for secondary stroke prevention. We set out to assess the sensitivity and specificity of the CHADS2 and CHA2DS2-VASc stroke risk scores as predictors of cardioembolic sources, documented by transesophageal echocardiography (TEE) in a population with ischemic stroke.
MethodsThe CHADS2 and CHA2DS2-VASc scores were applied to all patients admitted to the stroke unit/neurology ward of a Portuguese tertiary hospital with atrial fibrillation (diagnosed previously or during or after admission) who underwent TEE between January and August 2011.
The presence of a cardioembolic source was defined as the observation by TEE of spontaneous echo contrast in the left atrium and atrial appendage or thrombi in the left cardiac chambers.
ResultsWe studied 94 patients, 66.0% male, mean age 64.4 years (standard deviation 14.2). A cardioembolic source was detected in 20 patients. ROC curve analysis identified as predictors of cardioembolic source CHADS2 score ≥4 (sensitivity of 75.0%, specificity of 66.0%, p=0.014) and CHA2DS2-VASc score ≥5 (sensitivity of 83.3%, specificity of 58.0%, p=0.009).
ConclusionsBoth scores showed acceptable sensitivity as predictors of embolic risk in the context of secondary prevention of cardioembolic stroke. The CHA2DS2-VASc score has higher sensitivity than CHADS2 but lower specificity.
A cardioembolia representa uma das causas mais frequentes de lesões cerebrovasculares isquémicas, com prevalência estimada de 20-30% e implicações terapêuticas diretas que obrigam à sua correta avaliação. Apesar de a validação das escalas de risco cardioembólico (CHADS2 e, mais recentemente, CHA2DS2-VASc) em populações heterogéneas de doentes com fibrilhação furicular, desconhece-se ainda a sua validade em contexto de prevenção secundária cerebrovascular.
É objetivo deste trabalho estudar a sensibilidade e especificidade diferencial das escalas de risco cardioembólico como preditoras de fonte cardioembólica documentada por ecocardiograma transesofágico (ETE) numa população de doentes com AVC isquémico.
MétodosAplicámos as escalas CHADS2 e CHA2DS2-VASc a todos os doentes internados por evento cerebrovascular isquémico na Unidade de AVC/Enfermaria de Neurologia de um hospital central português com diagnóstico de fibrilhação auricular (prévio ou obtido durante/após o internamento), que realizaram ETE entre janeiro e agosto de 2011.
Definimos como presença de fonte cardioembólica a observação em ETE de autocontraste espontâneo na aurícula e apêndice auricular esquerdo ou trombos nas cavidades cardíacas esquerdas.
ResultadosAnalisámos 94 doentes, 66,0% do sexo masculino, idade média: 64,4 anos (desvio padrão: 14,2). Foi detetada fonte cardioembólica em 20 doentes. A análise de curva Receiver Operating Characteristic (ROC) identifica como preditores de fonte cardioembólica pontuação CHADS2 ≥ 4; sensibilidade: 75,0%, especificidade: 66,0%, p = 0,014 e pontuação CHA2DS2-VASc ≥ 5; sensibilidade: 83,3%, especificidade: 58,0%, p = 0,009.
ConclusõesAmbas as escalas apresentam sensibilidade significativa como preditoras de risco cardioembólico em contexto de prevenção secundária cerebrovascular. A escala CHA2DS2-VASc possui uma sensibilidade superior à CHADS2, sendo, no entanto, menos específica.
atrial fibrillation
area under the curve
confidence interval
electrocardiogram
international normalized ratio
patent foramen ovale
receiver operating characteristic
transesophageal echocardiography
Trial of Org 10172 in Acute Stroke Treatment
Stroke is the second leading cause of death worldwide,1 and has a particularly alarming prevalence in Portugal, where mortality from stroke is around 200/100 000 population, one of the highest in the European Union.2,3
Anatomopathologically and pathophysiologically, strokes are classified as ischemic (80%) or hemorrhagic (20%).4 Ischemic stroke is divided according to the TOAST (Trial of Org 10172 in Acute Stroke Treatment) etiological classification into five categories: large-artery atherosclerosis, cardioembolism, small-artery occlusion, stroke of other determined etiology, and stroke of undetermined etiology.5
A cardioembolic etiology is attributed to 20–30% of cases of ischemic stroke.6 Stroke risk is increased in a range of heart diseases, particularly atrial fibrillation (AF),7 but the risk of recurrent ischemic stroke in AF is significantly reduced by anticoagulant drugs for primary and secondary prevention.8,9
The CHADS2 score10 was developed to stratify the risk of cardioembolic stroke. Current guidelines relate CHADS2 score to the antithrombotic therapy to adopt11 and more recently, a new cardioembolic risk score, CHA2DS2-VASc, was created to refine the previous classification, but although these two scores have been validated in a heterogeneous population of AF patients for primary prevention,12,13 their accuracy has not been ascertained for secondary stroke prevention.
Transesophageal echocardiography (TEE) is an invasive exam that provides good anatomical information on the aortic arch, left atrium, left atrial appendage, and the mitral and aortic valves. It has high sensitivity and specificity for detecting cardioembolic sources, and is three times more accurate for this purpose than transthoracic echocardiography.14
The aim of this study was to analyze the possible correlation between the CHADS2 and CHA2DS2-VASc stroke risk scores and the presence of cardioembolic sources documented by TEE in patients with ischemic stroke.
MethodsPopulationWe included all patients admitted to the stroke unit/neurology ward of a Portuguese tertiary hospital with atrial fibrillation (diagnosed previously or during or after admission) who underwent TEE to investigate cardioembolic sources between January and August 2011.
TEE was performed up to five days after the vascular event in the cardiology ward of the same hospital using a GE Vivid 7 Dimension scanner with a 6Tc RS multiplane transesophageal probe. The presence of a cardioembolic source was defined as the observation on TEE of thrombi in the left cardiac chambers or spontaneous echo contrast in the left atrium and atrial appendage.15
Clinical variables (including hypertension, diabetes, coronary disease, congestive heart failure, smoking, dyslipidemia, personal history of stroke and alcohol abuse), demographic characteristics (age and gender), and laboratory results were obtained from patients’ medical records. The diagnosis of AF was based on direct observation of the relevant diagnostic exams. The efficacy of anticoagulation in previously anticoagulated patients (10; 10.6%) was assessed by the international normalized ratio (INR) on the date of the TEE study (±72 h).
All patients were classified according to the CHADS210 and CHA2DS2-VASc16 risk scores and were compared in terms of clinical and demographic variables and cardiovascular risk in order to analyze the possible correlation with the presence of cardioembolic sources.
Finally, we assessed the cutoffs, sensitivity, specificity and positive and negative predictive value of the two stroke risk scores as predictors of cardioembolic risk.
Statistical analysisIBM SPSS Statistics version 20 was used for the statistical analysis.
A descriptive analysis was performed, calculating means and standard deviation for quantitative variables and frequencies and percentages for qualitative variables.
Demographic variables and prevalence of vascular risk factors were compared between patients with and without a cardioembolic source, using the chi-square test or Fisher's exact test as appropriate for qualitative variables and the Student's t test for quantitative variables.
The different stroke scores and the date of TEE study were compared with the presence or absence of a cardioembolic source documented by TEE, using the Mann–Whitney U test for two independent variables.
Receiver operator characteristic (ROC) curves were constructed to determine the cutoffs to predict a cardioembolic source in the total population, assuming equal importance for sensitivity and specificity for each of the risk scores used. This analysis was then repeated for the subgroup of non-anticoagulated patients to assess the robustness of the results.
The level of statistical significance was set at p<0.05.
ResultsPopulationDuring the study period 313 patients were diagnosed with ischemic cerebrovascular events (stroke or transient ischemic attack). Of these, 94 (30.0%) with a diagnosis of AF underwent TEE study. AF had been diagnosed prior to admission in 66 (70.2%), during hospital stay in 25 (26.6%) and after discharge in 3 (3.2%). A cardioembolic source was identified in 20 patients (21.3%), detected by spontaneous echo contrast in the left cardiac chambers in 19 (20.2%), thrombi in the left cardiac chambers in one (1.1%) and both in eight (8.5%).
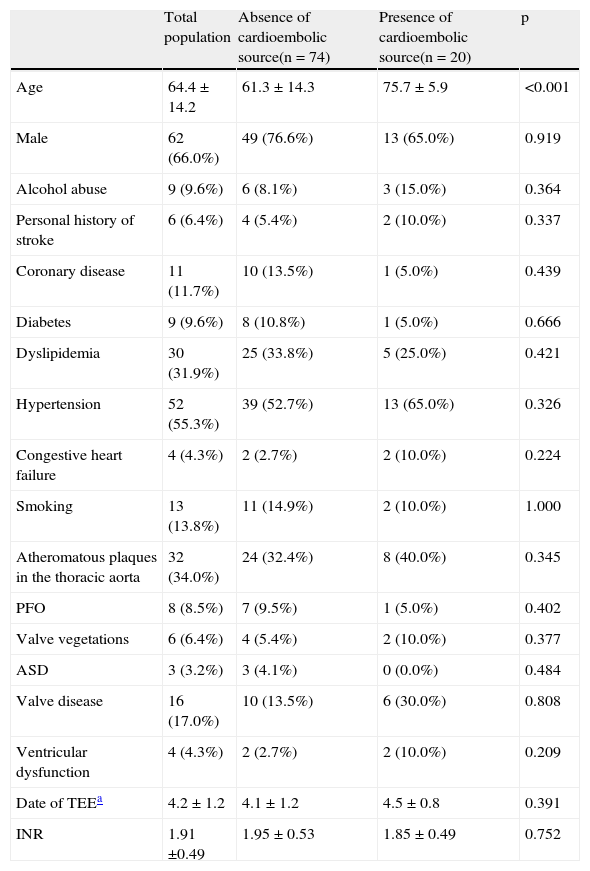
Table 1 shows the demographic characteristics, vascular risk factors and echocardiographic findings of the study population.
Prevalence of vascular risk factors in the study population.
| Total population | Absence of cardioembolic source(n = 74) | Presence of cardioembolic source(n = 20) | p | |
| Age | 64.4 ± 14.2 | 61.3 ± 14.3 | 75.7 ± 5.9 | <0.001 |
| Male | 62 (66.0%) | 49 (76.6%) | 13 (65.0%) | 0.919 |
| Alcohol abuse | 9 (9.6%) | 6 (8.1%) | 3 (15.0%) | 0.364 |
| Personal history of stroke | 6 (6.4%) | 4 (5.4%) | 2 (10.0%) | 0.337 |
| Coronary disease | 11 (11.7%) | 10 (13.5%) | 1 (5.0%) | 0.439 |
| Diabetes | 9 (9.6%) | 8 (10.8%) | 1 (5.0%) | 0.666 |
| Dyslipidemia | 30 (31.9%) | 25 (33.8%) | 5 (25.0%) | 0.421 |
| Hypertension | 52 (55.3%) | 39 (52.7%) | 13 (65.0%) | 0.326 |
| Congestive heart failure | 4 (4.3%) | 2 (2.7%) | 2 (10.0%) | 0.224 |
| Smoking | 13 (13.8%) | 11 (14.9%) | 2 (10.0%) | 1.000 |
| Atheromatous plaques in the thoracic aorta | 32 (34.0%) | 24 (32.4%) | 8 (40.0%) | 0.345 |
| PFO | 8 (8.5%) | 7 (9.5%) | 1 (5.0%) | 0.402 |
| Valve vegetations | 6 (6.4%) | 4 (5.4%) | 2 (10.0%) | 0.377 |
| ASD | 3 (3.2%) | 3 (4.1%) | 0 (0.0%) | 0.484 |
| Valve disease | 16 (17.0%) | 10 (13.5%) | 6 (30.0%) | 0.808 |
| Ventricular dysfunction | 4 (4.3%) | 2 (2.7%) | 2 (10.0%) | 0.209 |
| Date of TEEa | 4.2 ± 1.2 | 4.1 ± 1.2 | 4.5 ± 0.8 | 0.391 |
| INR | 1.91 ±0.49 | 1.95 ± 0.53 | 1.85 ± 0.49 | 0.752 |
ASD: atrial septal defect; INR: international normalized ratio; PFO: patent foramen ovale; TEE: transesophageal echocardiography.
At discharge 91 patients (96.8%) were under anticoagulant therapy, which was contraindicated in the other three (3.2%).
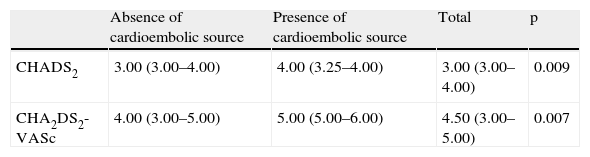
Cardioembolic risk and TEETable 2 shows the comparison between the CHADS2 and CHA2DS2-VASc scores and the presence or absence of a cardioembolic source documented by TEE.
Comparison between the two stroke risk scores and presence or absence of a cardioembolic source.
| Absence of cardioembolic source | Presence of cardioembolic source | Total | p | |
| CHADS2 | 3.00 (3.00–4.00) | 4.00 (3.25–4.00) | 3.00 (3.00–4.00) | 0.009 |
| CHA2DS2-VASc | 4.00 (3.00–5.00) | 5.00 (5.00–6.00) | 4.50 (3.00–5.00) | 0.007 |
Scores are presented as medians (interquartile range).
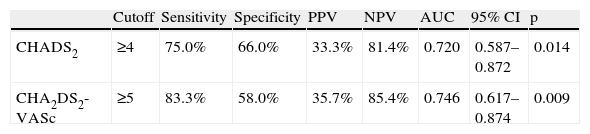
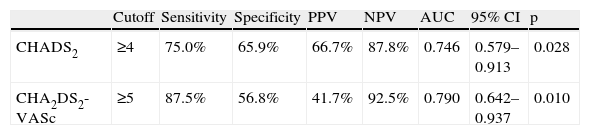
Analysis of the sensitivity, specificity and positive and negative predictive value of the CHADS2 and CHA2DS2-VASc risk scores is presented in Table 3 and Figure 1. Table 4 shows the results of the ROC curve analysis applied to the subgroup of non-anticoagulated patients (n = 84).
ROC curve analysis for the two stroke risk scores.
| Cutoff | Sensitivity | Specificity | PPV | NPV | AUC | 95% CI | p | |
| CHADS2 | ≥4 | 75.0% | 66.0% | 33.3% | 81.4% | 0.720 | 0.587–0.872 | 0.014 |
| CHA2DS2-VASc | ≥5 | 83.3% | 58.0% | 35.7% | 85.4% | 0.746 | 0.617–0.874 | 0.009 |
AUC: area under the curve; CI: confidence interval; NPV: negative predictive value; PPV: positive predictive value.
ROC curve analysis for the two stroke risk scores in non-anticoagulated patients only (n = 84).
| Cutoff | Sensitivity | Specificity | PPV | NPV | AUC | 95% CI | p | |
| CHADS2 | ≥4 | 75.0% | 65.9% | 66.7% | 87.8% | 0.746 | 0.579–0.913 | 0.028 |
| CHA2DS2-VASc | ≥5 | 87.5% | 56.8% | 41.7% | 92.5% | 0.790 | 0.642–0.937 | 0.010 |
AUC: area under the curve; CI: confidence interval; NPV: negative predictive value; PPV: positive predictive value.
The main aim of the present study was to assess the sensitivity and specificity of the CHADS2 and CHA2DS2-VASc stroke risk scores as predictors of cardioembolic sources in a population with ischemic stroke.
The results show that age is a major factor in the presence of cardioembolism, which is in agreement with other studies17 and reflects the increased importance of this factor in the more recent CHA2DS2-VASc score. No significant association was found between the other risk and demographic factors assessed and the presence of a cardioembolic source, which is also in line with previous studies.18 Mean INR values in previously anticoagulated patients were at subtherapeutic levels. There was no significant difference between those with and without a cardioembolic source; this should be interpreted with caution given the small number of patients treated with vitamin K antagonists, but it is a reminder of the need to maintain optimal dosages.
The variables used in this study to predict a cardioembolic source have been confirmed by various authors15,19,20 to be effective in AF patients. Although TEE is the best exam due to its ability to detect spontaneous contrast in the left atrium, in the light of current indications for anticoagulation therapy its routine use is not recommended for AF patients in the European Society of Cardiology guidelines, which reserve it for particular situations.21 However, in the acute phase of stroke, the attending physician often needs reliable information on cardioembolic risk in order to determine the timing of antithrombotic therapy, and for such decisions TEE findings can be crucial, even in patients previously diagnosed with AF.
Analysis of the CHADS2 and CHA2DS2-VASc scores in this study shows that this population is at moderate or high risk for stroke recurrence,22 which is to be expected.
The findings of the study clearly confirm the validity of both of these scores in classifying risk even in secondary stroke prevention. However, analysis of their sensitivity and specificity reveals differences, the more recent classification showing greater sensitivity in predicting a cardioembolic source but lower specificity than the earlier system. No differences were seen in positive or negative predictive value.
Various studies have shown that vitamin K antagonists are effective for primary and secondary stroke prevention, but they are also associated with bleeding complications and a significant percentage of patients discontinuing therapy.23 These disadvantages are among the reasons for the poor adherence to international guidelines for prevention of thromboembolic events.24 However, the development of new drugs that show equal or superior efficacy to warfarin in stroke prevention but with lower bleeding risk and without the need for regular laboratory testing25–28 has changed the risk/benefit ratio of prophylactic antithrombotic therapy in AF. It is now necessary to rethink the cutoffs for prescribing anticoagulation, and this requires risk scores with greater sensitivity in predicting ischemic events. The characteristics of the CHA2DS2-VASc scoring system as demonstrated in this study appear to indicate that it is more suitable for this task.
Study limitationsThe main limitations of this study are the fact that it was based on a single center and that TEE was not performed immediately after the vascular event, which raises the possibility that previously existing thrombi were not visualized by the exam. Furthermore, the frequently paroxysmal nature of AF means that the absence of a cardioembolic source at a particular time does not exclude its existence previously or subsequently.
ConclusionsThe findings of this study clearly confirm the validity of the CHADS2 and CHA2DS2-VASc scores in predicting cardioembolic risk in secondary stroke prevention. The more recent score, CHA2DS2-VASc, appears to be superior to the earlier scoring system, since the availability of new therapeutic approaches means that its slightly lower specificity is unlikely to compromise informed and individualized decision-making, and this is clearly outweighed by its higher sensitivity.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The first two authors contributed equally to this work.
Please cite this article as: Sá T, et al. CHADS2 e CHA2DS2VASc como preditores de fonte cardioembólica em prevenção secundária cerebrovascular. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.09.007.




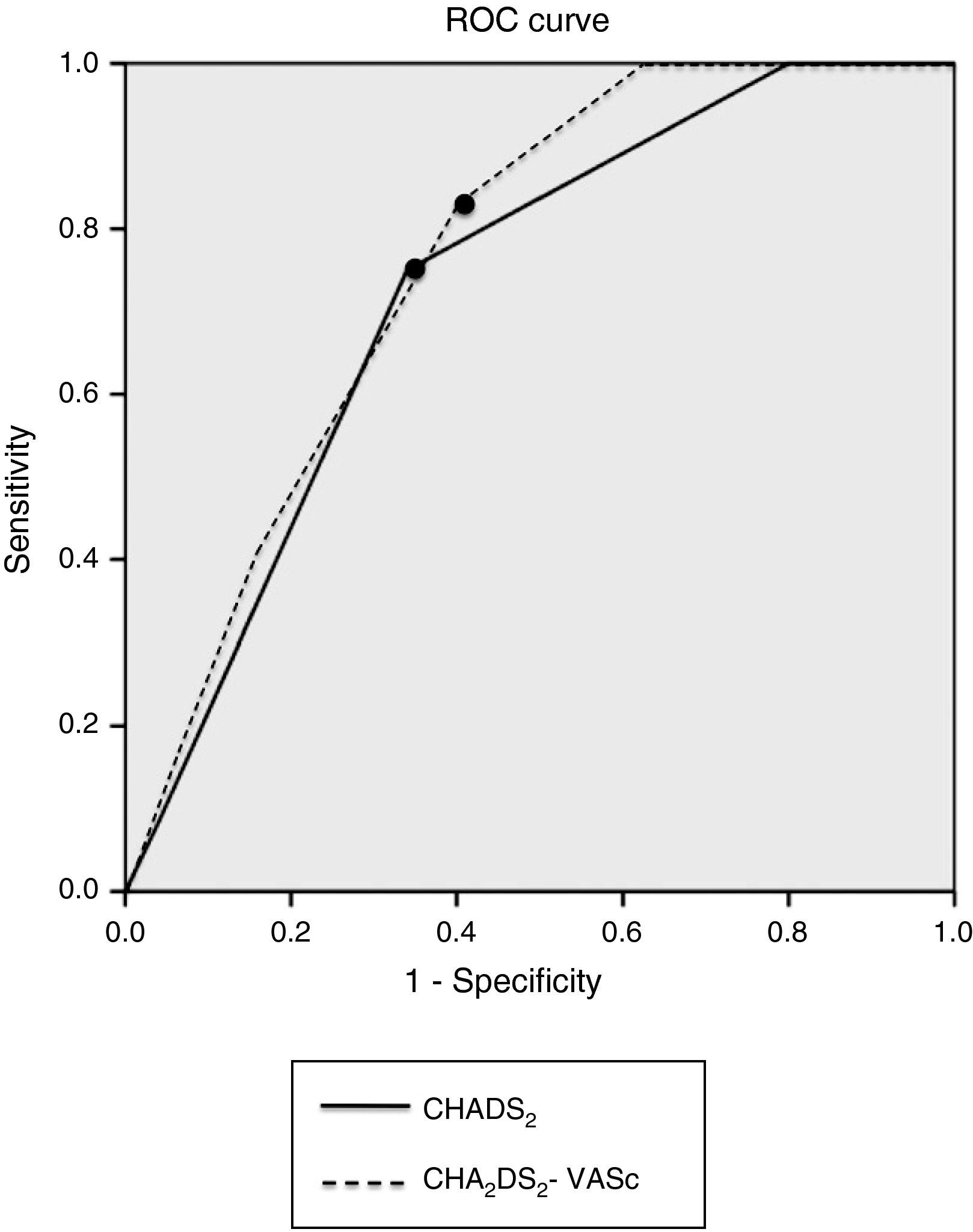
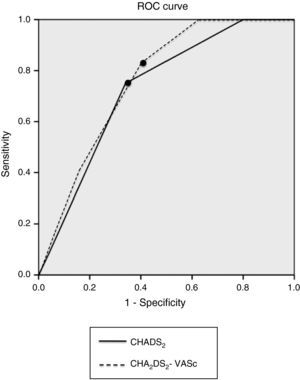 ROC curve analysis of sensitivity and specificity of the CHADS2 and CHA2DS2-VASc risk scores. The points indicate the cutoffs assuming equal importance for sensitivity and specificity and correspond to CHADS2 score ≥4 and CHA2DS2-VASc score ≥5.' title='
ROC curve analysis of sensitivity and specificity of the CHADS2 and CHA2DS2-VASc risk scores. The points indicate the cutoffs assuming equal importance for sensitivity and specificity and correspond to CHADS2 score ≥4 and CHA2DS2-VASc score ≥5.' title='
