The increasing use of anthracyclines, together with the longer survival of cancer patients, means the toxic effects of these drugs need to be monitored. In order to detect, prevent or mitigate anthracycline-induced cardiomyopathy, it is essential that all patients undergo a rigorous initial cardiovascular assessment, followed by close monitoring. Several clinical trials have shown the cardioprotective effect of non-pharmacological measures such as exercise, healthy lifestyles, control of risk factors and treatment of comorbidities; a cardioprotective effect has also been observed with pharmacological measures such as beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, statins, dexrazoxane and liposomal formulations. However, there are currently no guidelines for managing prevention in these patients. In this review the authors discuss the state of the art of the assessment, monitoring, and, above all, the prevention of anthracycline-induced cardiotoxicity.
O crescente uso de antraciclinas, aliado ao aumento da sobrevida dos doentes oncológicos, motiva a necessidade de monitorizar os efeitos tóxicos destes fármacos. Para que a sua cardiotoxicidade possa ser detetada, prevenida ou atenuada, torna-se essencial que todos os doentes sejam, do ponto de vista cardiovascular, submetidos a uma rigorosa avaliação inicial e a um estreito acompanhamento. Diversos ensaios clínicos comprovaram o efeito cardioprotetor produzido por medidas não farmacológicas como o exercício físico, a adoção de um estilo de vida saudável, o controlo de fatores de risco e o tratamento de comorbilidades; foi também verificado um efeito cardioprotetor com estratégias farmacológicas como o uso de bloqueadores-beta, inibidores da enzima de conversão da angiotensina, antagonistas do recetor da angiotensina, estatinas, dexrazoxane ou derivados lisossomais. No entanto, atualmente não existe qualquer diretriz científica que oriente as estratégias de prevenção nestes doentes. Com esta revisão propomo-nos abordar o estado da arte relativo à avaliação, monitorização e, principalmente, à prevenção da cardiotoxicidade provocada pelas antraciclinas.
According to the World Health Organization, cancer is the second leading cause of death worldwide.1 The considerable and ongoing advances in treatment have increased survival of cancer patients, but the adverse effects of chemotherapy, particularly on the heart, are a significant cause of mortality and morbidity. Mortality among cancer patients who develop anthracycline-induced cardiomyopathy is high (over 60% at two years),2 but prognosis can be improved by early detection and prevention.
Anthracyclines such as doxorubicin, daunorubicin, epirubicin, mitoxantrone and idarubicin are the most commonly used chemotherapy drugs in cancer. They are a known cause of cardiotoxicity (Table 1), with acute or/and subacute effects that can manifest as electrocardiographic changes, ventricular and supraventricular arrhythmias, cardiac conduction disturbances (atrioventricular or branch block), ventricular dysfunction, rises in brain-type natriuretic peptide (BNP, a marker of increased preload and heart failure [HF]), myocarditis and pericarditis, and that may occur at any time between beginning of treatment and two weeks after the end of treatment. These effects are relatively uncommon and most revert a week after treatment cessation. Chronic cardiomyopathy is defined as early if it begins within a year of ending chemotherapy and late after that period. In either case, systolic or diastolic dysfunction are observed (Table 2) that can progress to severe cardiomyopathy and may even lead to death.3 Although some studies have suggested that the risk of developing ventricular dysfunction and its severity can be predicted on the basis of acute myocardial injury,4 the relationship between acute and chronic toxicity is not fully understood. Diagnosis of cardiac dysfunction induced by cancer therapy has been the subject of various studies,3,5 one of which5 is considered the reference publication on the subject, and is based on HF symptoms, physical examination and parameters of left ventricular function.
Cardiotoxicity, pharmacokinetics and therapeutic use of anthracyclines.
| Mechanisms of action | Mechanisms of cardiotoxicity | Anthracycline | Therapeutic use | Cardiotoxicity |
|---|---|---|---|---|
| The formation of a DNA complex by conjugation of flat rings with nucleotides inhibits DNA and RNA and protein synthesis. This triggers DNA cleavage by topoisomerase II, resulting in cytotoxicity. Anthracyclines inhibit helicase, preventing enzymatic cleavage of the DNA double strand and thus interfering with replication and transcription. They cause redox reactions through formation of cytotoxic free radicals. | Main mechanisms: - topoisomerase II beta-mediated DNA damage - lipid peroxidation - oxidative stress - apoptosis and necrosis of cardiac cells Impaired synthesis of DNA, RNA and proteins and of transcription factors involved in regulation of genes specific to the heart. Negative balance of sarcomeric proteins in cardiac cells caused by reduced protein expression and increased myofilament degradation. Combination therapy exacerbates myofilament loss. Mitochondrial DNA damage and changes in mitochondrial bioenergetics. Disruption of the dynamic regulation of cardiac function, altering adrenergic and adenylyl cyclase activity and calcium homeostasis. | Doxorubicin | Advanced stomach cancer Bladder cancer Breast cancer Ovarian cancer Small cell lung cancer Thyroid cancer Hodgkin disease Acute leukemia Non-Hodgkin lymphoma Neuroblastoma Sarcoma Wilms tumor | Acute: Hypotension Arrhythmias Tachycardia Thromboembolism Subacute: Pericarditis Myocarditis Chronic: Dilated cardiomyopathy Contractile dysfunction Congestive heart failure |
| Daunorubicin | Acute lymphoblastic leukemia Acute myeloid leukemia | Acute: Sinus tachycardia Tachyarrhythmias Ventricular extrasystoles AV block Chronic: Dilated cardiomyopathy Contractile dysfunction Congestive heart failure | ||
| Epirubicin | Advanced ovarian cancer Stomach cancer Breast cancer Lung cancer | Acute: Ventricular tachycardia AV block Bundle branch block Bradycardia Thromboembolism Chronic: Dilated cardiomyopathy Contractile dysfunction Congestive heart failure | ||
| Idarubicin | Acute lymphocytic leukemia Acute myeloid leukemia | Acute: Arrhythmias Atrial fibrillation Myocardial infarction Thromboembolism Chronic: Dilated cardiomyopathy Contractile dysfunction Congestive heart failure | ||
| Mitoxantrone | Advanced breast cancer Acute myeloid leukemia in adults Non-Hodgkin lymphoma | Acute: Arrhythmias Myocarditis Hypertension Myocardial ischemia Chronic: Dilated cardiomyopathy Contractile dysfunction Congestive heart failure |
AV: atrioventricular.
Adapted from 24,58,59.
Criteria to confirm or revise a preliminary diagnosis of chemotherapy-induced cardiac dysfunction, according to the Cardiac Review and Evaluation Committee.
| Any one of the criteria is sufficient to confirm a diagnosis of cardiac dysfunction. |
| Cardiomyopathy characterized by a decrease in cardiac LVEF that is either global or more severe in the septum |
| Symptoms of CHF |
| Detection of S3 gallop, tachycardia, or both; |
| Decline in LVEF of at least 5% to less than 55% with accompanying signs or symptoms of CHF, or a decline in LVEF of at least 10% to below 55% without accompanying signs or symptoms. |
CHF: congestive heart failure; LVEF: left ventricular ejection fraction.
Adapted from 3,60.
One proposed classification divides chemotherapy-induced cardiomyopathy into two types: type I, caused by anthracyclines, which induce irreversible dose-dependent cardiac injury; and type II, caused by trastuzumab, which is not related to the cumulative dose and is often reversible after treatment discontinuation.6 The second type will not be discussed in this review article.
In this review the authors discuss strategies in patients being treated with anthracyclines in order to prevent or mitigate their main adverse effects on the heart.
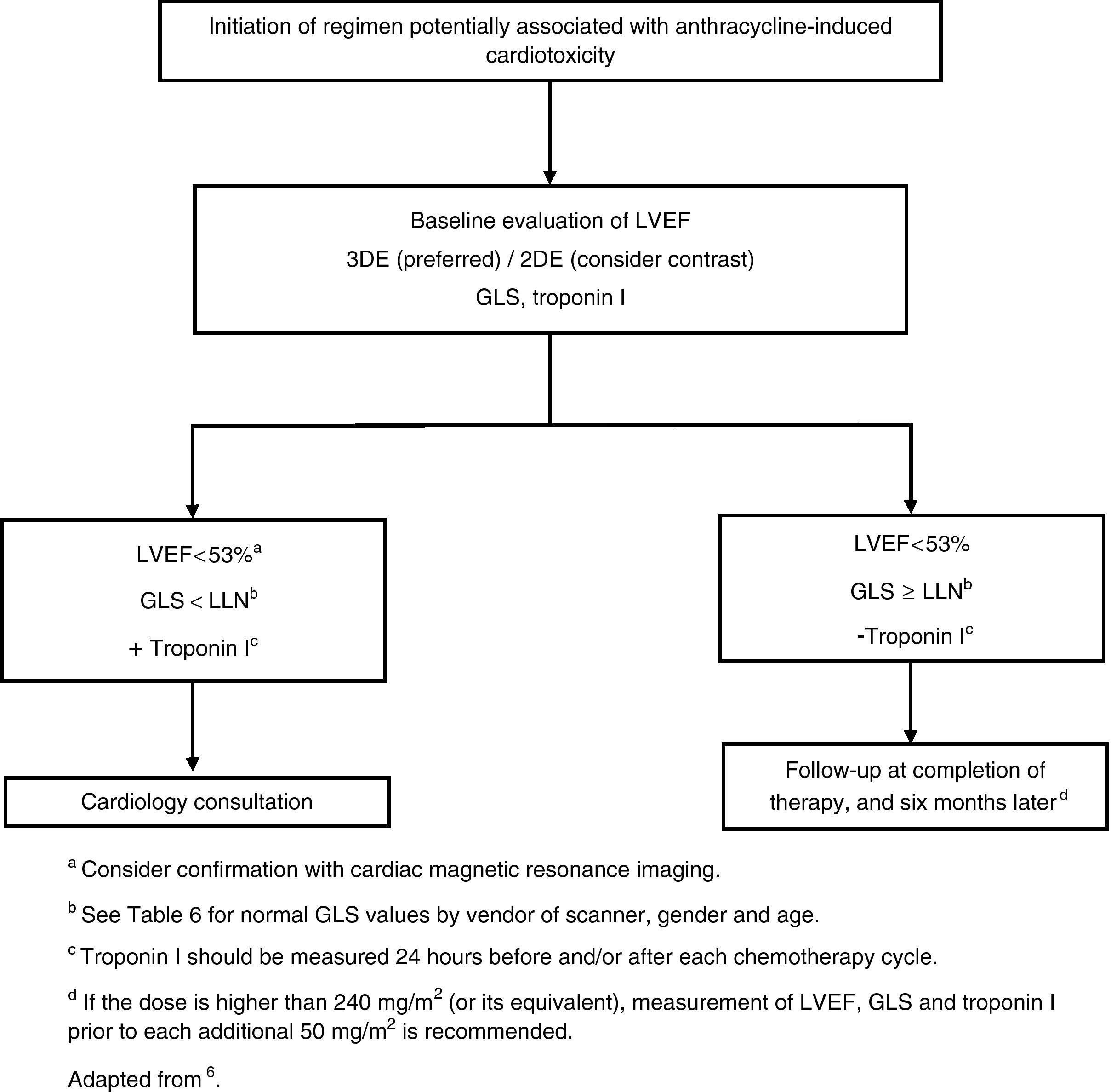
Initial assessmentIn view of the cardiotoxicity of anthracyclines, all patients referred for chemotherapy should undergo a cardiac assessment to establish their baseline cardiovascular characteristics, which can then be used during the treatment regimen for purposes of comparison. This assessment should include clinical history and physical examination, electrocardiography to determine cardiac rhythm and detect signs of ischemia, and cardiac imaging, usually transthoracic echocardiography with complete Doppler study (Tables 3 and 4). When the echocardiogram is provides insufficient information, cardiac magnetic resonance imaging (CMRI) is recommended. Baseline troponin levels should also be measured for future comparisons.5
Recommended cardio-oncology echocardiogram protocol.
| Standard transthoracic echocardiography |
| • In accordance with ASE/EAE guidelines and IAC-Echo |
| 2D strain imaging acquisition |
| • Apical 3-, 4-, and 2-chamber views |
| • Acquire ≥3 cardiac cycles |
| • Images obtained simultaneously maintaining the same 2D frame rate and imaging depth |
| Frame rate between 40 and 90 frames/s or ≥40% of HR |
| • Aortic VTI (aortic ejection time) |
| 2D strain imaging analysis |
| • Quantify segmental and global longitudinal strain |
| • Display the segmental strain curves from apical views in a quad format |
| • Display the global strain in a bull's-eye plot |
| 2D strain imaging pitfalls |
| • Ectopy |
| • Breathing translation |
| 3D imaging acquisition |
| • Apical 4-chamber full volume to assess LV volumes and to calculate LVEF |
| • Single and multiple beats optimizing spatial and temporal resolution |
| Reporting |
| • Timing of echocardiography with respect to the IV infusion (number of days before or after) |
| • Vital signs (BP, HR) |
| • 3D LVEF/2D biplane Simpson method |
| • GLS (echocardiography machine, software, and version used) |
| • In the absence of GLS, measurement of medial and lateral s′ and MAPSE |
| • RV: TAPSE |
Adapted from 5.
2D: two-dimensional; 3D: three-dimensional; ASE/EAE: American Society of Echocardiography/European Association of Echocardiography; BP: blood pressure; GLS: global longitudinal strain; HR: heart rate; IAC-Echo: Intersocietal Accreditation Commission Echocardiography; IV: intravenous; LV: left ventricular; LVEF: left ventricular ejection fraction; MAPSE: mitral annular plane systolic excursion; RV: right ventricle; TAPSE: tricuspid annular plane systolic excursion; VTI: velocity-time integral.
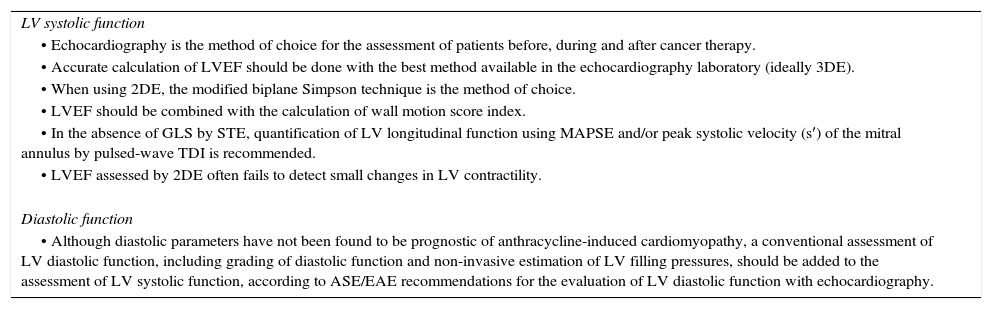
Echocardiographical assessment of systolic and diastolic function in the cancer patient.
| LV systolic function |
| • Echocardiography is the method of choice for the assessment of patients before, during and after cancer therapy. |
| • Accurate calculation of LVEF should be done with the best method available in the echocardiography laboratory (ideally 3DE). |
| • When using 2DE, the modified biplane Simpson technique is the method of choice. |
| • LVEF should be combined with the calculation of wall motion score index. |
| • In the absence of GLS by STE, quantification of LV longitudinal function using MAPSE and/or peak systolic velocity (s′) of the mitral annulus by pulsed-wave TDI is recommended. |
| • LVEF assessed by 2DE often fails to detect small changes in LV contractility. |
| Diastolic function |
| • Although diastolic parameters have not been found to be prognostic of anthracycline-induced cardiomyopathy, a conventional assessment of LV diastolic function, including grading of diastolic function and non-invasive estimation of LV filling pressures, should be added to the assessment of LV systolic function, according to ASE/EAE recommendations for the evaluation of LV diastolic function with echocardiography. |
Adapted from 5.
2DE: two-dimensional echocardiography; 3DE: three-dimensional echocardiography; ASE/EAE: American Society of Echocardiography/European Association of Echocardiography; GLS: global longitudinal strain; LV: left ventricular; LVEF: left ventricular ejection fraction; STE: speckle-tracking echocardiography; TDI: tissue Doppler imaging.
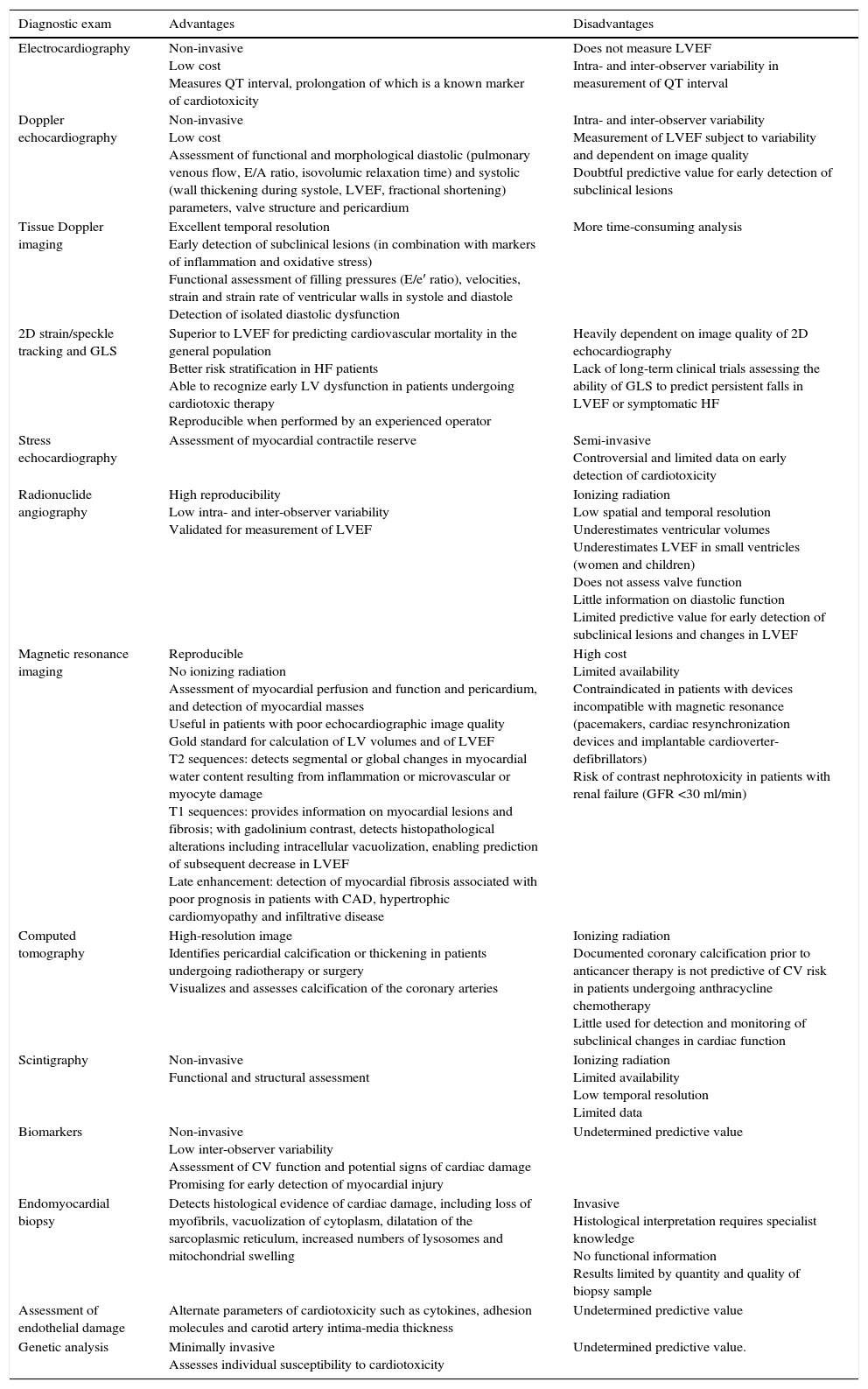
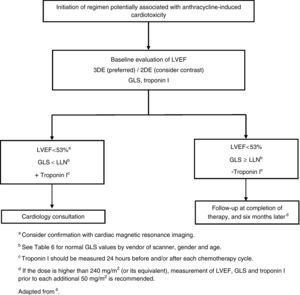
It is important to monitor for signs and symptoms of cardiotoxicity during chemotherapy (Table 5). The 12-lead electrocardiogram can be used routinely to screen for arrhythmias due to anthracycline-related cardiotoxicity, while 24-hour Holter monitoring or an event recorder can be useful to investigate the etiology of syncope presumed to result from arrhythmia or advanced atrioventricular block.7 Cardiac function should be monitored by echocardiography in patients under anthracycline therapy. Global longitudinal strain (GLS) as assessed by two-dimensional speckle tracking is a more sensitive predictor of HF than left ventricular ejection fraction (LVEF),8 since during anthracycline therapy changes in GLS precede reduction in LVEF.5 However, in clinical practice, fractional shortening and LVEF have been the most widely used parameters,9 although fractional shortening is proving to be less reliable in this context. These parameters, being dependent on pre- and afterload, are less sensitive for early detection of preclinical cardiac disease. Various studies have suggested that assessment of diastolic function by Doppler echocardiography may enable early detection of anthracycline-induced cardiomyopathy.10,11 If LVEF is <53%, GLS below the limit of normal (Table 6), and/or troponins are elevated, a cardiology consultation should be considered, with discussion between the cardiologist and oncologist of the risk/benefit ratio of chemotherapy.5 A fall in LVEF during anthracycline therapy is associated with increased risk for cardiac events, and although a reduction in GLS of <8% compared to baseline appears not to be significant, a reduction of >15% is likely to indicate cardiotoxicity.5 The study should be repeated two to three weeks after the baseline study to confirm the diagnosis. CMRI can detect subtle changes in the myocardium and increases in extracellular volume, which suggest edema or diffuse fibrosis. Although it is highly sensitive and reproducible for assessment of cardiac function and characterization of myocardial tissue, CMRI has the disadvantages of limited availability and high cost.12 Radionuclide angiography is reproducible and more easily available, but exposes patients to ionizing radiation, increasing their cumulative dose, especially when serial studies are required, and provides only limited information on diastolic function and valve morphology, and so should not be the method of choice.12 Endomyocardial biopsy has greater sensitivity and specificity for detection and monitoring of the adverse effects of anthracyclines,5 enabling visualization of loss of myofibrils, vacuolization of cytoplasm, dilatation of the sarcoplasmic reticulum, increased numbers of lysosomes and mitochondrial swelling.12 However, the invasive nature of the procedure limits its use in clinical practice. Biomarkers have been validated in various studies; they are specific not only in detecting cardiovascular injury but also in determining its extent and reversibility. While troponin T and I are indicators of cardiomyocyte damage, BNP and the N-terminal portion of pro-BNP (NT-proBNP) reflect increased myocardial stress.13,14 According to the literature, elevation of troponins is an early indicator of cardiotoxicity, while BNP is less consistent.
Advantages and disadvantages of diagnostic exams in the assessment of anthracycline-induced cardiotoxicity.
| Diagnostic exam | Advantages | Disadvantages |
|---|---|---|
| Electrocardiography | Non-invasive Low cost Measures QT interval, prolongation of which is a known marker of cardiotoxicity | Does not measure LVEF Intra- and inter-observer variability in measurement of QT interval |
| Doppler echocardiography | Non-invasive Low cost Assessment of functional and morphological diastolic (pulmonary venous flow, E/A ratio, isovolumic relaxation time) and systolic (wall thickening during systole, LVEF, fractional shortening) parameters, valve structure and pericardium | Intra- and inter-observer variability Measurement of LVEF subject to variability and dependent on image quality Doubtful predictive value for early detection of subclinical lesions |
| Tissue Doppler imaging | Excellent temporal resolution Early detection of subclinical lesions (in combination with markers of inflammation and oxidative stress) Functional assessment of filling pressures (E/e′ ratio), velocities, strain and strain rate of ventricular walls in systole and diastole Detection of isolated diastolic dysfunction | More time-consuming analysis |
| 2D strain/speckle tracking and GLS | Superior to LVEF for predicting cardiovascular mortality in the general population Better risk stratification in HF patients Able to recognize early LV dysfunction in patients undergoing cardiotoxic therapy Reproducible when performed by an experienced operator | Heavily dependent on image quality of 2D echocardiography Lack of long-term clinical trials assessing the ability of GLS to predict persistent falls in LVEF or symptomatic HF |
| Stress echocardiography | Assessment of myocardial contractile reserve | Semi-invasive Controversial and limited data on early detection of cardiotoxicity |
| Radionuclide angiography | High reproducibility Low intra- and inter-observer variability Validated for measurement of LVEF | Ionizing radiation Low spatial and temporal resolution Underestimates ventricular volumes Underestimates LVEF in small ventricles (women and children) Does not assess valve function Little information on diastolic function Limited predictive value for early detection of subclinical lesions and changes in LVEF |
| Magnetic resonance imaging | Reproducible No ionizing radiation Assessment of myocardial perfusion and function and pericardium, and detection of myocardial masses Useful in patients with poor echocardiographic image quality Gold standard for calculation of LV volumes and of LVEF T2 sequences: detects segmental or global changes in myocardial water content resulting from inflammation or microvascular or myocyte damage T1 sequences: provides information on myocardial lesions and fibrosis; with gadolinium contrast, detects histopathological alterations including intracellular vacuolization, enabling prediction of subsequent decrease in LVEF Late enhancement: detection of myocardial fibrosis associated with poor prognosis in patients with CAD, hypertrophic cardiomyopathy and infiltrative disease | High cost Limited availability Contraindicated in patients with devices incompatible with magnetic resonance (pacemakers, cardiac resynchronization devices and implantable cardioverter-defibrillators) Risk of contrast nephrotoxicity in patients with renal failure (GFR <30 ml/min) |
| Computed tomography | High-resolution image Identifies pericardial calcification or thickening in patients undergoing radiotherapy or surgery Visualizes and assesses calcification of the coronary arteries | Ionizing radiation Documented coronary calcification prior to anticancer therapy is not predictive of CV risk in patients undergoing anthracycline chemotherapy Little used for detection and monitoring of subclinical changes in cardiac function |
| Scintigraphy | Non-invasive Functional and structural assessment | Ionizing radiation Limited availability Low temporal resolution Limited data |
| Biomarkers | Non-invasive Low inter-observer variability Assessment of CV function and potential signs of cardiac damage Promising for early detection of myocardial injury | Undetermined predictive value |
| Endomyocardial biopsy | Detects histological evidence of cardiac damage, including loss of myofibrils, vacuolization of cytoplasm, dilatation of the sarcoplasmic reticulum, increased numbers of lysosomes and mitochondrial swelling | Invasive Histological interpretation requires specialist knowledge No functional information Results limited by quantity and quality of biopsy sample |
| Assessment of endothelial damage | Alternate parameters of cardiotoxicity such as cytokines, adhesion molecules and carotid artery intima-media thickness | Undetermined predictive value |
| Genetic analysis | Minimally invasive Assesses individual susceptibility to cardiotoxicity | Undetermined predictive value. |
Adapted from 5,12,61.
2D: two-dimensional; CAD: coronary artery disease; CV: cardiovascular; ECG: electrocardiography; GFR: glomerular filtration rate; GLS: global longitudinal strain; HF: heart failure; LV: left ventricular; LVEF: left ventricular ejection fraction.
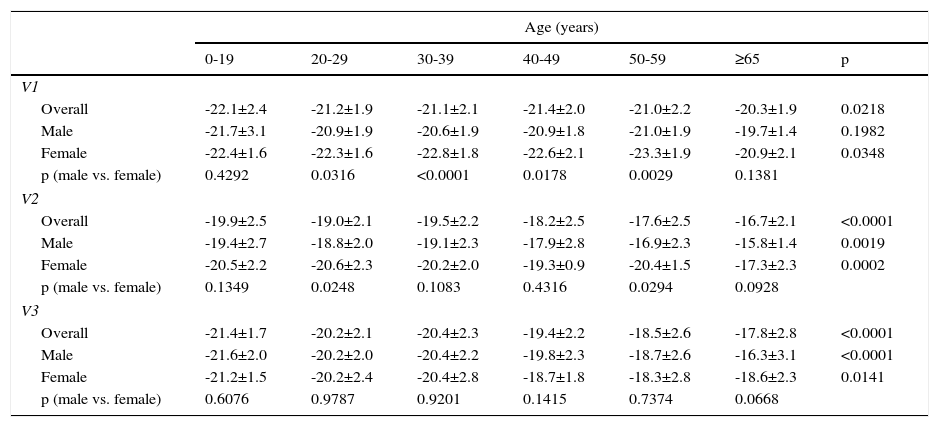
Normal values of global longitudinal strain by vendor of scanner, gender, and age.
| Age (years) | |||||||
|---|---|---|---|---|---|---|---|
| 0-19 | 20-29 | 30-39 | 40-49 | 50-59 | ≥65 | p | |
| V1 | |||||||
| Overall | -22.1±2.4 | -21.2±1.9 | -21.1±2.1 | -21.4±2.0 | -21.0±2.2 | -20.3±1.9 | 0.0218 |
| Male | -21.7±3.1 | -20.9±1.9 | -20.6±1.9 | -20.9±1.8 | -21.0±1.9 | -19.7±1.4 | 0.1982 |
| Female | -22.4±1.6 | -22.3±1.6 | -22.8±1.8 | -22.6±2.1 | -23.3±1.9 | -20.9±2.1 | 0.0348 |
| p (male vs. female) | 0.4292 | 0.0316 | <0.0001 | 0.0178 | 0.0029 | 0.1381 | |
| V2 | |||||||
| Overall | -19.9±2.5 | -19.0±2.1 | -19.5±2.2 | -18.2±2.5 | -17.6±2.5 | -16.7±2.1 | <0.0001 |
| Male | -19.4±2.7 | -18.8±2.0 | -19.1±2.3 | -17.9±2.8 | -16.9±2.3 | -15.8±1.4 | 0.0019 |
| Female | -20.5±2.2 | -20.6±2.3 | -20.2±2.0 | -19.3±0.9 | -20.4±1.5 | -17.3±2.3 | 0.0002 |
| p (male vs. female) | 0.1349 | 0.0248 | 0.1083 | 0.4316 | 0.0294 | 0.0928 | |
| V3 | |||||||
| Overall | -21.4±1.7 | -20.2±2.1 | -20.4±2.3 | -19.4±2.2 | -18.5±2.6 | -17.8±2.8 | <0.0001 |
| Male | -21.6±2.0 | -20.2±2.0 | -20.4±2.2 | -19.8±2.3 | -18.7±2.6 | -16.3±3.1 | <0.0001 |
| Female | -21.2±1.5 | -20.2±2.4 | -20.4±2.8 | -18.7±1.8 | -18.3±2.8 | -18.6±2.3 | 0.0141 |
| p (male vs. female) | 0.6076 | 0.9787 | 0.9201 | 0.1415 | 0.7374 | 0.0668 | |
V1: Vivid 7 or Vivid E9 (GE Healthcare); V2: iE33 (Philips Medical Systems); V3: Artida or Apilo (Toshiba Medical Systems).
Adapted from 62.
If the dose of anthracyclines exceeds 240 mg/m2, cardiac assessment should be repeated before administering further cycles (Figure 1).
Prevention of cardiotoxicityPrevention of anthracycline-induced cardiotoxicity, while maintaining the drugs’ therapeutic effectiveness, can be achieved by pharmacological and non-pharmacological means.
Non-pharmacological preventionCardiovascular risk factors should be identified and treated appropriately as soon as cancer is diagnosed. Patients should be encouraged to adopt a healthy lifestyle, including a diet low in saturated fat and a maximum of 2.5 g of sodium per day, avoid toxic substances such as alcohol and tobacco, and maintain their body mass index close to 25 kg/m2. Exercise, whether of low or high intensity, during anthracycline therapy increases cardiovascular reserve15 and studies in animal models have indicated that it may reduce the cardiotoxic effects of these agents.16 Although exercise has shown promise in improving cardiopulmonary function in breast cancer survivors,17,18 there have been no clinical trials in humans that confirm their cardioprotective role. Another measure is to reduce or avoid the use of drugs that prolong QT interval, particularly 5-hydroxytryptamine 3 antagonists (frequently used to prevent adverse effects of chemotherapy including nausea and vomiting) and antihistamines.19,20 It is also important to minimize radiation exposure, to correct electrolyte disturbances and to treat comorbidities (Table 7).21
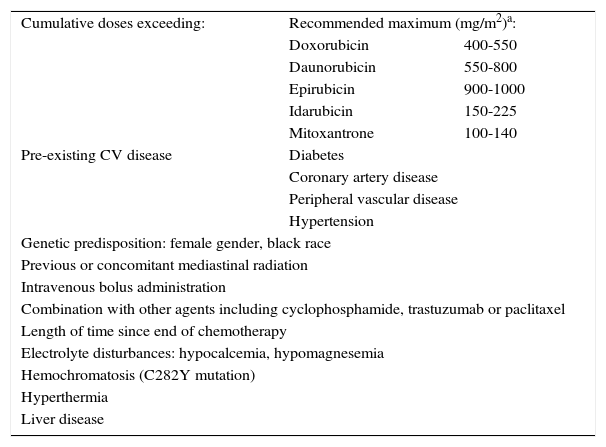
Risk factors for anthracycline-induced cardiotoxicity.
| Cumulative doses exceeding: | Recommended maximum (mg/m2)a: | |
| Doxorubicin | 400-550 | |
| Daunorubicin | 550-800 | |
| Epirubicin | 900-1000 | |
| Idarubicin | 150-225 | |
| Mitoxantrone | 100-140 | |
| Pre-existing CV disease | Diabetes | |
| Coronary artery disease | ||
| Peripheral vascular disease | ||
| Hypertension | ||
| Genetic predisposition: female gender, black race | ||
| Previous or concomitant mediastinal radiation | ||
| Intravenous bolus administration | ||
| Combination with other agents including cyclophosphamide, trastuzumab or paclitaxel | ||
| Length of time since end of chemotherapy | ||
| Electrolyte disturbances: hypocalcemia, hypomagnesemia | ||
| Hemochromatosis (C282Y mutation) | ||
| Hyperthermia | ||
| Liver disease | ||
CV: cardiovascular.
Patients aged over 65 and children may develop cardiotoxicity with lower cumulative doses.
Adapted from 12,24.
Decreasing the dose of anthracyclines is another way to reduce the incidence of left ventricular systolic dysfunction (LVSD), as shown by a study of patients taking 400, 500 or 550 mg/m2 of doxorubicin, in which the incidence of congestive HF was 5%, 16% and 26% respectively.22 Although anthracyclines appear to be cardiotoxic independently of the dose administered, several studies have shown that continuous infusion of lower doses for between 24 and 92 hours23 can reduce the severity of cardiac injury, and has been described as an effective way of doing so.24 Prolonging infusion time reduces cardiotoxicity without compromising the effectiveness of chemotherapy,25 but infusion lasting longer than 96 hours is associated with a high incidence of stomatitis. The only case in which continuous infusion of doxorubicin appears to have no cardioprotective effect compared to rapid infusion is in children with acute lymphoblastic leukemia (ALL).26 Other clinical trials using endomyocardial biopsy to assess anthracycline-induced cardiac injury in different drug regimens concluded that continuous perfusion leads to far less significant damage than rapid intravenous administration.27 These trials also showed that patients receiving continuous infusion had greater tolerance for higher cumulative doses of doxorubicin. Although animal studies demonstrated that anthracycline levels in tumor tissue were the same however the drugs were administered (continuous or rapid infusion), this was not true of cardiac tissue, in which rapid infusion led to higher concentrations and thus greater toxicity.28
Pharmacological preventionAntioxidantsAlthough antioxidants neutralize free radicals formed by anthracycline therapy and thus theoretically reduce or prevent cardiotoxicity, clinical trials of N-acetylcysteine, coenzyme Q, L-carnitine, phenethylamines, amifostine and a combination of vitamins E and C and N-acetylcysteine did not show a cardioprotective effect.29 Erythropoietin and iloprost30 have been shown to protect against the cardiotoxic effects of doxorubicin in vitro, without affecting its anticancer effectiveness, but their cardioprotective ability will have to be demonstrated in vivo.
Liposomal formulationsOne way to combat the adverse cardiac effects of anthracyclines is to change the formulation of the drugs such as encapsulating them in liposomes.31 Studies comparing unencapsulated and liposome-encapsulated doxorubicin found no difference in anti-tumor response rate, overall survival or progression-free survival, but the incidence of HF and LVSD was lower in patients treated with the liposomal formulation, and this group also had a lower incidence of other adverse effects including neutropenia, nausea, vomiting and diarrhea.32 Due to their high cost, these formulations are not widely used and the US Food and Drug Administration (FDA) has approved their use only for ovarian cancer, AIDS-related Kaposi's sarcoma, and patients with multiple myeloma who have not responded to a year of treatment with other drugs.33
DexrazoxaneAdministration of dexrazoxane concomitantly with anticancer regimens can have a cardioprotective effect, preventing elevation of troponins and reducing the incidence of HF.34 Some authors attribute the cardioprotective effect of this iron chelator to its reduction of the quantity of intracellular iron, which may decrease doxorubicin-induced free radical generation.34 However, studies on other iron chelators have not demonstrated cardioprotection.35,36 It has also been suggested that dexrazoxane's cardioprotective effect is due not only to its antagonizing topoisomerase II cleavage complex formation, but also to its induction of rapid degradation of topoisomerase II beta, which suggests that this enzyme is involved in anthracycline-induced cardiotoxicity.37 A study of dexrazoxane in over 200 children with ALL showed that it reduced troponin T elevation in both sexes,38 and limited reduction of fractional shortening and maintained left ventricular thickness-to-dimension ratio at five years, but only in girls.39 Recently three cases have been reported of adults undergoing chemotherapy combined with dexrazoxane for breast cancer who developed acute myeloid leukemia. However, two studies comparing dexrazoxane with placebo in children with ALL followed for five and 10 years showed no difference in the incidence of secondary malignancy.40,41 Nevertheless, in view of its known adverse effects, the FDA and the European Medicines Agency have restricted the use of dexrazoxane to adult patients with advanced or metastatic breast cancer who have already received a cumulative dose of doxorubicin of more than 300 mg/m2 and who will benefit from additional anthracycline therapy.42
Beta-blockersThe cardioprotection afforded by beta-blockers (BBs) appears to derive from their antioxidant and anti-apoptotic properties. One BB, carvedilol, has shown particular promise in reducing the incidence of anthracycline-induced cardiomyopathy and preserving systolic and diastolic function.43 In children, carvedilol limited troponin I elevation and improved both fractional shortening and peak global systolic strain.44 According to some studies, BBs and angiotensin-converting enzyme (ACE) inhibitors (see below) can prevent the remodeling associated with HF by reducing adrenergic response.45,46 However, no cardioprotective effects have been seen with either metoprolol or enalapril.47
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockersACE inhibitors and angiotensin receptor blockers (ARBs) show cardioprotective properties, possibly by reducing oxidative stress, left ventricular remodeling, and apoptosis.48 When administered for at least two years after discontinuation of chemotherapy in children with anthracycline-induced LVSD, enalapril showed no benefit in terms of reducing left ventricular end-systolic wall stress or preserving fractional shortening.49 By contrast, in adults treated with high-dose anthracyclines, enalapril prevented HF and worsening of parameters of cardiac function such as LVEF.50 Studies in angiotensin II type I receptor knockout mice showed that doxorubicin did not have a cardiotoxic effect in these animals and that the administration of ARBs can prevent daunorobucin-induced cardiomyopathy.48 Although to our knowledge there have been only two randomized trials on ARBs in chemotherapy patients, valsartan was shown to prevent acute prolongation of corrected QT, left ventricular diastolic dilatation and elevation of BNP during one week of chemotherapy, although with no effect on LVEF,51 and telmisartan prevented reduction in peak strain rate during high-dose anthracycline therapy.52 Further studies are required with longer follow-up to confirm these effects.
StatinsStatins have antioxidant and anti-inflammatory properties.53 Studies in animal models demonstrate that fluvastatin mitigates anthracycline-induced cardiotoxicity, reducing oxidative stress and enhancing the expression of the antioxidant enzyme mitochondrial superoxide dismutase 2, resulting in reduced cardiac inflammation.53 In one clinical trial assessing the effect of continuous statin treatment in patients with breast cancer receiving anthracycline-based chemotherapy, patients receiving statins had a lower incidence of HF.54 In another study in patients with previously normal LVEF undergoing anthracycline chemotherapy, LVEF was unchanged at six months in those treated with atorvastatin, compared to a fall of 8% in the control group.55
Finally, it should be emphasized that there is as yet no solid evidence for the effectiveness of pharmacological prevention of anthracycline-induced cardiomyopathy, and so the main preventive strategy remains thorough prior cardiovascular assessment of patients and appropriate monitoring, selection and adjustment of chemotherapy dosages.
Treatment of heart failureAfter the development of signs or symptoms of HF or a reduction in LVEF due to chemotherapy-related cardiotoxicity, treatment should be based on the current guidelines.56 Although selection of the best therapy is obviously important, one study has shown that the main factor determining successful treatment is the time between the end of chemotherapy and the start of HF therapy, since if this is longer than six months, LVEF is unlikely to recover completely.57
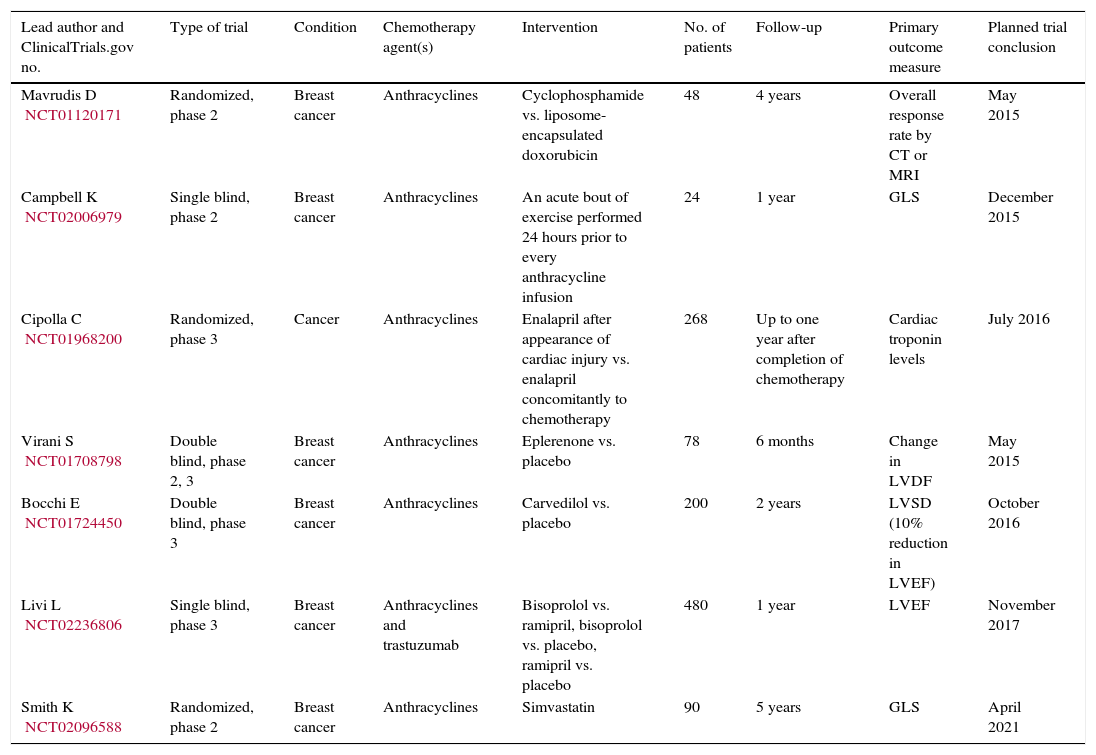
Prospects for the futureSeveral clinical trials are currently under way aiming to assess various therapeutic strategies, pharmacological and non-pharmacological, for the prevention of anthracycline-induced cardiomyopathy (Table 8). It will be some years before the results are known, and there is still a pressing need for evidence-based guidelines for the assessment and clinical monitoring of these patients.
Clinical trials on prevention of anthracycline-induced cardiomyopathy.
| Lead author and ClinicalTrials.gov no. | Type of trial | Condition | Chemotherapy agent(s) | Intervention | No. of patients | Follow-up | Primary outcome measure | Planned trial conclusion |
|---|---|---|---|---|---|---|---|---|
| Mavrudis D NCT01120171 | Randomized, phase 2 | Breast cancer | Anthracyclines | Cyclophosphamide vs. liposome-encapsulated doxorubicin | 48 | 4 years | Overall response rate by CT or MRI | May 2015 |
| Campbell K NCT02006979 | Single blind, phase 2 | Breast cancer | Anthracyclines | An acute bout of exercise performed 24 hours prior to every anthracycline infusion | 24 | 1 year | GLS | December 2015 |
| Cipolla C NCT01968200 | Randomized, phase 3 | Cancer | Anthracyclines | Enalapril after appearance of cardiac injury vs. enalapril concomitantly to chemotherapy | 268 | Up to one year after completion of chemotherapy | Cardiac troponin levels | July 2016 |
| Virani S NCT01708798 | Double blind, phase 2, 3 | Breast cancer | Anthracyclines | Eplerenone vs. placebo | 78 | 6 months | Change in LVDF | May 2015 |
| Bocchi E NCT01724450 | Double blind, phase 3 | Breast cancer | Anthracyclines | Carvedilol vs. placebo | 200 | 2 years | LVSD (10% reduction in LVEF) | October 2016 |
| Livi L NCT02236806 | Single blind, phase 3 | Breast cancer | Anthracyclines and trastuzumab | Bisoprolol vs. ramipril, bisoprolol vs. placebo, ramipril vs. placebo | 480 | 1 year | LVEF | November 2017 |
| Smith K NCT02096588 | Randomized, phase 2 | Breast cancer | Anthracyclines | Simvastatin | 90 | 5 years | GLS | April 2021 |
CT: computed tomography; GLS: global longitudinal strain; LVDF: left ventricular diastolic function; LVEF: left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; MRI: magnetic resonance imaging.
The longer survival of patients undergoing anticancer therapy and the consequent increase in the incidence of anthracycline-induced cardiomyopathy mean that it is necessary to investigate and determine the precise mechanisms leading to adverse cardiac effects, in order to prevent them. Further research will enable specific and validated prevention plans to be established.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cruz M, Duarte-Rodrigues J, Campelo M. Cardiotoxicidade na terapêutica com antraciclinas: estratégias de prevenção. Rev Port Cardiol. 2016;35:359–371.