Left ventricular pseudoaneurysm is a rare complication of acute myocardial infarction, associated with high mortality. However, it can present in a non-specific manner, complicating and delaying the diagnosis.
The authors present the case of a 65-year-old patient, hypertensive, with no other known relevant medical history, who presented with chest pain, cough and left pleural effusion, initially attributed to a pulmonary process. However, these were in fact the result of a left ventricular pseudoaneurysm following silent acute myocardial infarction. The diagnosis was suspected on echocardiography and confirmed by cardiac magnetic resonance imaging, and the patient underwent successful surgical pseudoaneurysm repair.
This case illustrates an atypical presentation of a left ventricular pseudoaneurysm, in which the manifestations resulted from pericardial and pleural extension of the inflammatory process associated with contained myocardial rupture. The case demonstrates the need for a high index of suspicion, and the value of imaging techniques to confirm it, in order to proceed with appropriate surgical treatment, and thus modify the course of the disease.
O pseudoaneurisma do ventrículo esquerdo é uma complicação rara do enfarte agudo do miocárdio, associada a elevada mortalidade. No entanto, pode manifestar-se de modo inespecífico, dificultando e atrasando o seu diagnóstico.
Os autores apresentam o caso de um doente de 65 anos, hipertenso, sem outros antecedentes relevantes conhecidos, em que toracalgia, tosse e derrame pleural esquerdo, inicialmente atribuídas a um processo pneumológico, foram as manifestações de um pseudoaneurisma do ventrículo esquerdo, após enfarte agudo do miocárdio silencioso. O diagnóstico foi suspeitado por ecocardiografia, confirmado por ressonância magnética cardíaca e o doente foi submetido a cirurgia de reparação do pseudoaneurisma com sucesso.
Este caso ilustra uma forma de apresentação atípica de um pseudoaneurisma do ventrículo esquerdo, em que as manifestações resultaram da extensão pericárdico-pleural do processo inflamatório associada à rotura miocárdica contida. O caso demonstra a necessidade de suspeitar o diagnóstico e o valor dos vários exames de imagem para a confirmação do mesmo, de modo a possibilitar a terapêutica cirúrgica adequada e assim modificar o curso da doença.
Left ventricular pseudoaneurysm is a rare mechanical complication of acute myocardial infarction that results from myocardial rupture contained by the adjacent pericardium, and is associated with high mortality. It can develop in individuals without previous clinical events and have a non-specific and insidious presentation. Surgical repair can modify the course of the disease, and so prompt diagnosis is of considerable clinical importance.1,2
Case reportThe authors present the case of a 65-year-old man, hypertensive, who was admitted to the emergency department with pleuritic left chest pain and nonproductive cough that had progressively worsened over the previous three months. He was feverish, tachycardic, and polypneic, with reduced breath sounds in the lower third of the left hemithorax. There were no signs of pulmonary congestion, abnormalities on cardiac auscultation, or peripheral edema. Laboratory tests revealed leukocytosis 11000/μl, C-reactive protein 10.50 mg/dl and troponin <0.02 μg/ml. The chest X-ray showed a slight opacity of the lower third of the left lung, suggesting pneumonia with pleural effusion. This possibility was supported by thoracic computed tomography (CT), which revealed a left pleural effusion and thickening of the pleural membranes with high contrast uptake, suggesting an inflammatory process associated with atelectasis or possible consolidation of the adjacent pulmonary parenchyma. Thoracentesis produced a liquid with the appearance of exudate, but cultures were negative. Other microbiological studies were also negative, including cultures from blood, expectorate, bronchoalveolar lavage and transbronchial pulmonary biopsy. Despite multiple courses of antibiotics, including tuberculostatics, the patient's fever persisted and inflammatory markers remained elevated.
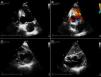
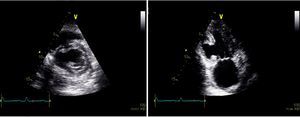
From the time of admission, the patient presented pathological Q waves and negative T waves in III and aVF on the electrocardiogram (ECG), suggesting inferior myocardial scarring. Transthoracic echocardiography showed deformation of the inferior wall, expanding in systole, and apparent reduction in wall thickness, together with a small pericardial effusion, suggesting a pseudoaneurysm rather than a true aneurysm (Figure 1 and Video 1).
Transthoracic echocardiograms suggestive of pseudoaneurysm of the inferior wall (arrow). (A and B) Apical 2-chamber view shows aneurysm of the inferior wall with reduced wall thickness and color Doppler suggesting flow between the ventricle and the aneurysm; transverse section, parasternal short-axis view in basal (C) and subcostal (D) planes. Small pericardial effusion (*).
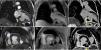
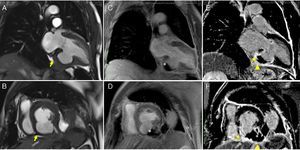
Cardiac magnetic resonance imaging (MRI) confirmed a small pericardial effusion, without hemodynamic compromise, with increased signal intensity in the pericardial space in perfusion sequences, thrombotic material adhering to the walls in early and late enhancement sequences and pericardial late enhancement. These findings confirmed the diagnosis of pseudoaneurysm of the mid and basal segments of the inferior wall, partially filled with thrombotic material (Figure 2 and Video 2).
Cardiac magnetic resonance imaging. Steady-state free precession sequences in 2-chamber long-axis (A) and short-axis (B) view of the left ventricle, revealing pseudoaneurysm of the inferior wall (arrowhead) and a small pericardial effusion; (C and D) early enhancement images showing evidence of thrombus (*) partially filling the pseudoaneurysm; transmural late enhancement involving the basal and mid segments of the inferior septum and the mid segment of the lateral wall. The basal inferior segment of the left ventricular wall is composed of thrombus (*), infarct scar (arrow) and pericardium (arrowhead); (E and F) late enhancement is clearly seen on the pericardial membrane, confirming the diagnosis of pseudoaneurysm.
Coronary angiography revealed an 80% lesion in the proximal circumflex artery and occlusion of the mid segment of the right coronary artery.
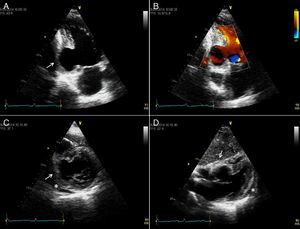
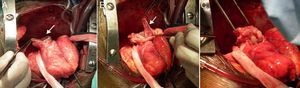
The patient underwent surgery, which showed a large pseudoaneurysm with a thick fibrous neck, containing thrombotic material and extending to the left inferior wall and pericardium. It was repaired using the modified Dor procedure (exclusion of the pseudoaneurysm and placement of a Dacron patch) and aortocoronary venous bypass grafting to the first obtuse marginal, which were uneventful (Figure 3). During the operation the patch was seen to be slightly above the neck, which was very wide. The patient's clinical course was favorable, with remission of chest pain, fever, inflammatory markers and pleural effusion. Control echocardiography showed persisting aneurysm of the inferior wall affecting various components of the ventricular wall (Figure 4 and Video 3).
Left ventricular pseudoaneurysm results from myocardial rupture in which the bleeding is contained by the pericardium, preventing the development of cardiac tamponade. The periphery of the hematoma organizes into fibrous tissue adhering to the adjacent pericardium, forming a saccular structure with no muscle tissue, containing organized thrombi and communicating with the ventricular chamber.1–3
Transmural myocardial infarction is the most common cause of left ventricular pseudoaneurysm (55%). Less frequent causes include complications of cardiac surgery (33%), chest trauma (7%), endocarditis (5%), and, rarely, as a consequence of purulent pericarditis or tumor infiltration.1,4
Pseudoaneurysms are usually located in the inferior or posterior walls, following occlusion of the right coronary or circumflex artery. By contrast, 80-90% of true aneurysms are located in the apex or anterolateral wall, due to occlusion of the anterior descending artery.
The surgical technique is generally similar for true aneurysms and pseudoaneurysms when repair is indicated due to their size or complications. However, true aneurysms contain muscle tissue, making rupture extremely unlikely, unlike pseudoaneurysms. Differential diagnosis between the two entities is thus essential, although not always easy.5
The risk of sudden death through pseudoaneurysm rupture is 30-45%. A significant proportion of patients are probably not diagnosed in the acute phase due to early and fatal rupture.2 The condition is mostly diagnosed in the chronic phase and can develop for many years without rupturing; around 10% of cases are completely asymptomatic. There may be symptoms of heart failure due to left ventricular systolic dysfunction resulting from a large pseudoaneurysm, as well as mitral regurgitation arising from dysfunction of the posterior papillary muscle and/or changes in the morphology of the left ventricle caused by the pseudoaneurysm.2,4 Chest pain may be due to post-infarction myocardial ischemia or to pericardial inflammation resulting from rupture, as appears to have been the case in our patient. There may also be arrhythmias or systemic embolism of thrombotic material from the pseudoaneurysm.4
Clinical diagnosis can be difficult, as clinical manifestations and physical exam tend to be non-specific. The ECG and chest X-ray may also be inconclusive, further hampering diagnosis.6,7
In the case presented, chest pain accompanied by cough and fever in a patient without relevant history did not raise suspicions of a pseudoaneurysm. Moreover, left pleural effusion with exudative characteristics, although mentioned in several case reports in the literature, led the diagnostic investigation further in the wrong direction.4,8 It was the electrocardiographic alterations that led to the suspicion of a cardiogenic cause, and imaging studies were crucial in confirming the diagnosis of pseudoaneurysm.
Echocardiography, with or without contrast, CT, MRI and ventriculography can establish the diagnosis, differentiating pseudoaneurysm from true aneurysm.3
Echocardiography is the first-line modality for identifying aneurysmal dilatation, raising the suspicion of discontinuity in the ventricular endocardium, and detecting a relatively narrow opening between the ventricular and aneurysmal cavities through which systolic and diastolic flow can be observed. Although echocardiography detects abnormalities in up to 90% of patients, it can only provide a definitive diagnosis in 25-33% of cases.9
The value of cardiac MRI has been established for characterizing pseudoaneurysms and identifying adjacent structures, particularly the pericardium, thrombi and discontinuity in the myocardium, enabling differential diagnosis with true aneurysm.6 Perfusion study is particularly useful when there is pericardial effusion as shown by increased signal intensity, suggesting communication with the ventricular chamber. Furthermore, as mentioned in the literature and seen in the case presented, late enhancement of the adjacent pericardium due to the presence of blood components can help in diagnosing a contained rupture.10
Although ventriculography has been considered the method of choice for the diagnosis of pseudoaneurysm, more recently the advantages of imaging methods such as echocardiography and MRI have been increasingly recognized, since they are non-invasive and do not involve the use of nephrotoxic contrast or ionizing radiation.2,7
For this reason, after the diagnosis in the case presented was confirmed by MRI, it was decided not to perform ventriculography at the time of coronary angiography.
As the risk of fatal rupture of pseudoaneurysms in the acute phase of myocardial infarction is very high (50%), surgical repair is indicated in all cases, even though this is also associated with high mortality (23-28%).1,4
There is disagreement concerning the best treatment of chronic cases. It has been suggested that even long-lasting pseudoaneurysms are indicated for surgery, since the risk of rupture and death tends to increase over time, and there is also a risk of cerebral thromboembolism (10%/year).1,2 However, small series of patients have shown 90% survival at one year and 75% at four years, and indicate that the risk of sudden death is proportional to the severity of heart failure, degree of left ventricular dysfunction and extent of underlying coronary disease. The risk of rupture is also higher in the first months after infarction and in cases of rapid expansion.11,12 According to this reasoning, surgical repair should be reserved for symptomatic patients, those diagnosed recently (<3 months), and those with large (>3 cm) or progressively expanding pseudoaneurysms.4
In our case, the patient presented with a pseudoaneurysm of undetermined evolution and with significant symptoms for around three months, basically caused by pericardial inflammation, the inflammatory process extending to the pleura and resulting in a significant effusion. It was therefore decided to perform urgent surgical repair of the pseudoaneurysm.
ConclusionPleuritic chest pain, cough, and signs of an ongoing inflammatory process led the diagnostic investigation to focus wrongly on a pulmonary process, until it finally became clear that these phenomena were merely due to extension of pericardial and pleural inflammation arising from myocardial rupture.
Few and non-specific signs and symptoms have also been observed in other cases reported in the literature, which highlights the need for a high index of suspicion. Imaging techniques are crucial to establish the diagnosis, and the information they provide is essential for early and appropriate surgical intervention.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Faustino M, Ranchordás S, Abecasis J, et al. Pseudoaneurisma ventricular esquerdo – um desafio diagnóstico. Rev Port Cardiol. 2016;35:373.e1–373e6.