Transcatheter aortic valve implantation (TAVI) is considered an alternative therapy in high-risk patients with severe aortic stenosis. Although a minimally invasive procedure, it is not free from complications, one of which is valve embolization at the time of TAVI. We present a case of embolization of a balloon-expandable aortic valve due to sigmoid left ventricular hypertrophy and managed with a second valve without surgery. The embolized valve was repositioned in the aortic arch between the left common carotid artery and the brachiocephalic trunk.
A implantação percutânea valvular aórtica (TAVI) é considerada uma terapêutica alternativa nos doentes de alto risco com estenose aórtica grave. Apesar de se tratar de um procedimento minimamente invasivo, o mesmo não está livre de complicações. Uma das quais é a embolização valvular no momento da TAVI. Apresentamos um caso de embolização valvular aórtica expansível por balão devido a hipertrofia ventricular esquerda sigmoide tratada com uma segunda válvula sem cirurgia. A válvula embolizada foi reposicionada na crossa da aorta entre a artéria carótida esquerda e o tronco braquiocefálico.
Transcatheter aortic valve implantation (TAVI) is emerging as an alternative to surgical aortic valve replacement for patients with symptomatic severe aortic stenosis considered to be at high or prohibitive operative risk.1 We report a case of embolization of an Edwards SAPIEN aortic valve due to sigmoid left ventricular hypertrophy, and discuss potential causes and solutions.
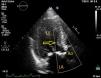
Case reportA 75-year-old woman diagnosed with severe symptomatic aortic stenosis was referred for TAVI. The patient had a history of diabetes, hypertension, coronary artery disease, morbid obesity, and chronic renal failure. Transthoracic echocardiography (TTE) showed sigmoid left ventricular hypertrophy and ejection fraction of 60%, and Doppler echocardiography revealed a mean aortic gradient of 50 mmHg and an aortic valve area of 0.96 cm2 (Figure 1). Transesophageal echocardiography (TEE) carried out for detailed examination revealed sigmoid left ventricular hypertrophy and an aortic annulus diameter of 24 mm. The aortic annulus diameter measured 26 mm×22 mm on multislice computed tomography. The patient was considered to be at too high risk for surgical aortic valve replacement and was referred for TAVI by a transfemoral approach.
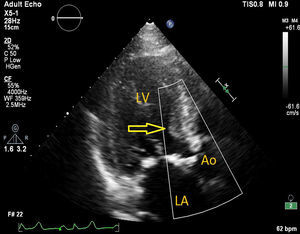
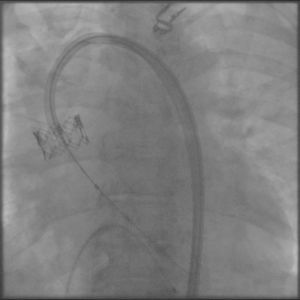
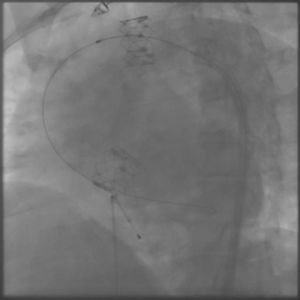
The process was performed under deep anesthesia and transthoracic echocardiographic guidance. The TAVI approach was through the right femoral artery using a 26 mm Edwards SAPIEN valve (Edwards Lifesciences, Inc., CA, USA). During balloon inflation under rapid pacing the valve prosthesis immediately embolized into the ascending aorta (Figure 2). Attempts to position the valve in the descending aorta were unsuccessful and the bioprosthesis was re-expanded into the aortic arch between the brachiocephalic trunk and the left common carotid artery. At this stage a second Edwards SAPIEN valve was successfully implanted with gradual balloon inflation (Figure 3). Arch aortography was carried out which showed no aortic regurgitation and no evidence of obstruction of the left common carotid artery or brachiocephalic trunk. The patient was transferred to the intensive care unit in a hemodynamically stable condition and was discharged one week after the procedure.
DiscussionTAVI is a rapidly emerging treatment option for high-risk and inoperable patient groups. However, this new therapeutic modality raises new questions and problems that need to be identified and resolved. Although less invasive than open-chest aortic valve replacement, TAVI is associated with potentially serious complications, such as valve embolization. Valve embolization during TAVI is a life-threatening complication that requires immediate diagnosis and treatment.
Procedural embolization of percutaneously implanted valves has been previously reported, with an incidence of 1.01%.2 Makkar et al. showed that patients with valve embolization had a greater body surface area and were more likely to be male. A cause for embolization was defined in post-procedural operator reports in 73% of cases. The most commonly stated causes were malpositioning, complex annulus/aortic valve anatomy, and pacing failure. Other causes included post-cardiopulmonary resuscitation, post-dilation, cardiac manipulation, displacement during attempted transcatheter valve-in-valve therapy, poor fluoroscopic angle for implantation, and incomplete/delayed device balloon inflation.2
In our case, distal embolization occurred due to sigmoid left ventricular hypertrophy, an example of complex annulus/aortic valve anatomy. In implantation of an aortic valve via a percutaneous route, the key factors for proper placement and fixation are choice of an appropriate size valve, accurate alignment and correct positioning. One of the most important limitations to the use of the Edwards SAPIEN valve system is sigmoid septum, which can lead to embolization of the prosthesis. In patients with pronounced sigmoid septum, apical placement of the Edwards SAPIEN valve or use of a Medtronic CoreValve are recommended.3 However, the presence of severe left ventricular hypertrophy and sigmoid septum are also an important predictor for permanent pacemaker requirement after the use of the CoreValve system. In this case, we preferred to use the Edwards SAPIEN valve because of lack of experience in apical placement and the Medtronic CoreValve system. Outcomes of distal aortic embolization of the Edwards SAPIEN valve remain good. The embolized prosthesis may be repositioned into the aortic arch without need for removal or surgery.4,5
Finally, the precise positioning of the valve, appropriate valve selection, and the route of administration of the procedure appear to be crucial for reducing the risk of valve migration.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.