The anaerobic threshold (AT) is an objective and direct measurement that reflects variations in metabolism of skeletal muscles during exercise. Its prognostic value in heart disease of non-Chagas etiology is well established. The risk of mortality in Chagas cardiomyopathy is relatively well assessed by the Rassi score. However, the added value that AT can bring to the Rassi score has not been studied.
ObjectiveTo assess whether AT presents additional prognostic value to the Rassi score in patients with chronic Chagas cardiomyopathy.
MethodsIn this prospective dynamic cohort study, 150 medical records were reviewed, and 45 records of patients who underwent cardiopulmonary exercise testing between 1996 and 1997 and followed until September 2015 were selected. The data were analyzed using a logistic regression model to detect associations between the study variables. The fit of the models was confirmed using receiver operating curves and the coefficient of determination R2.
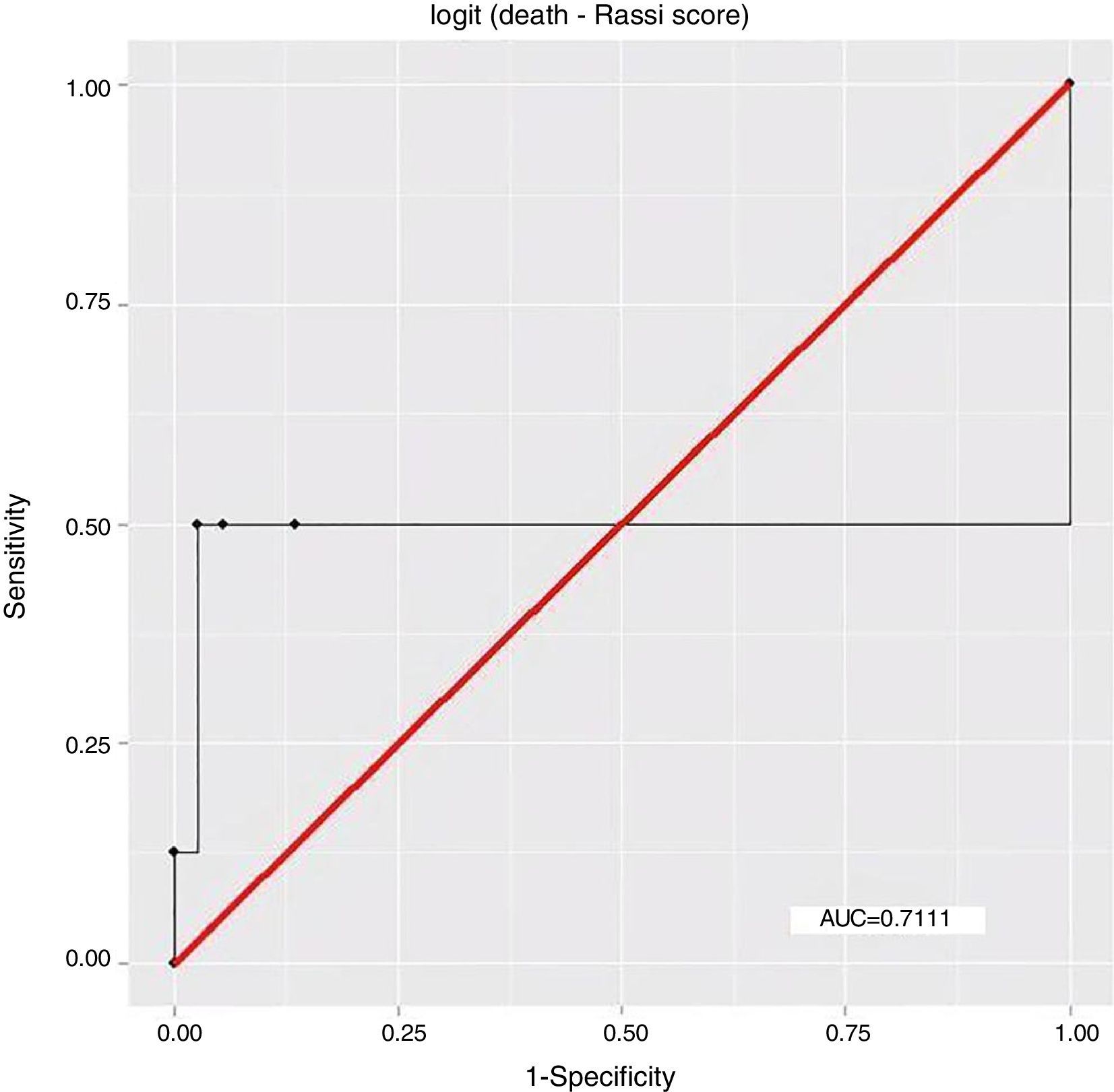
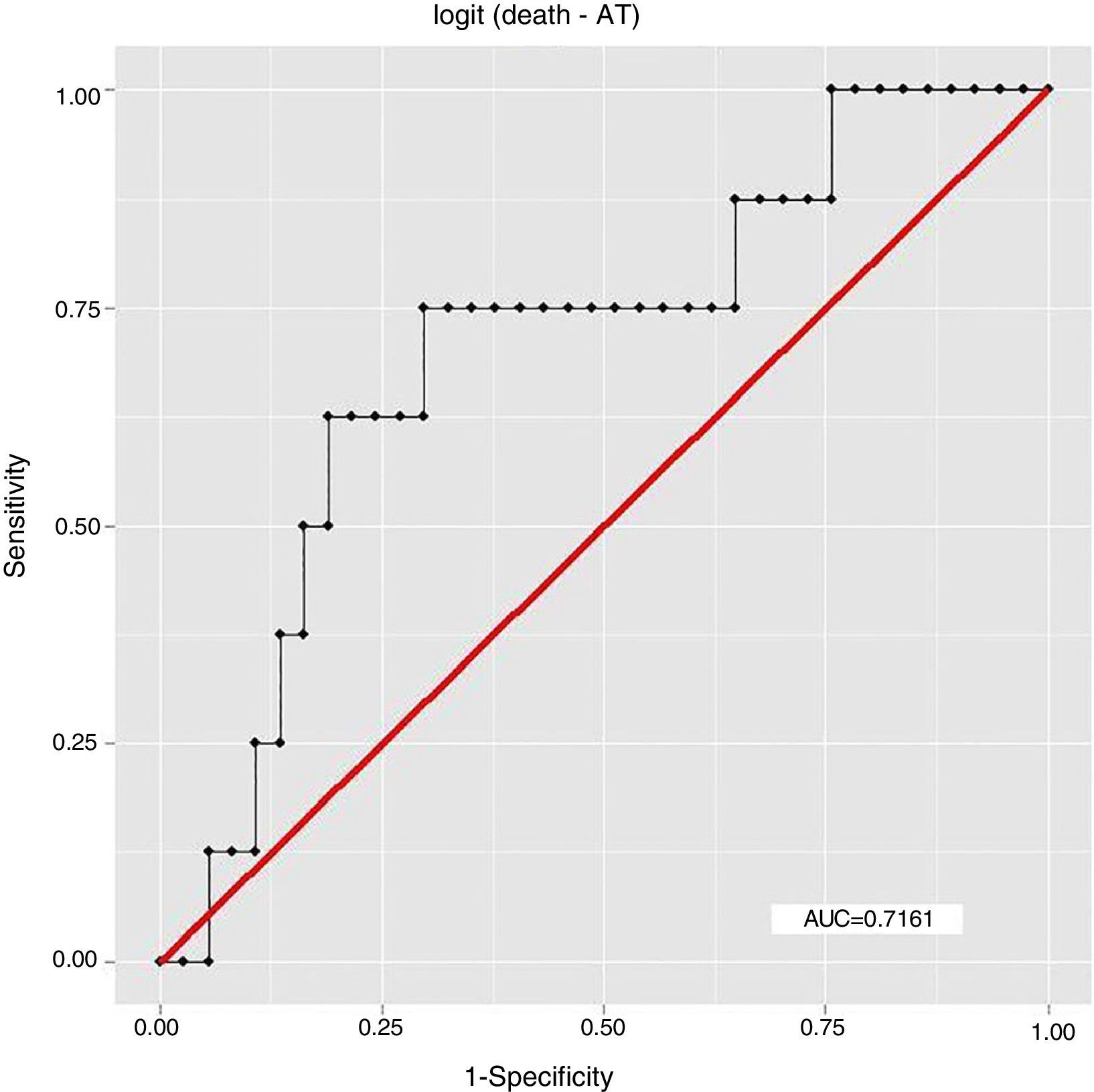
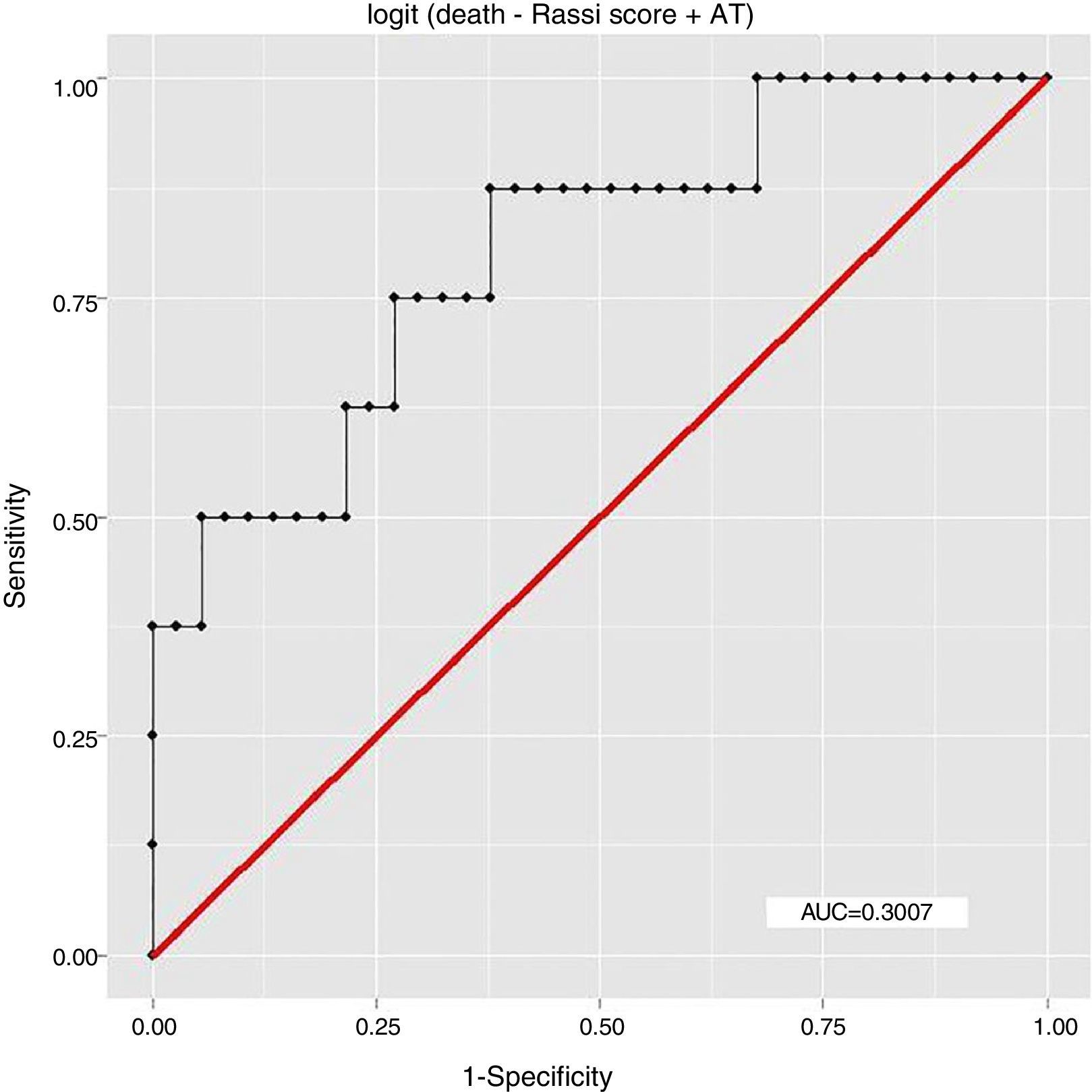
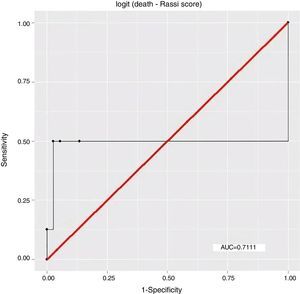
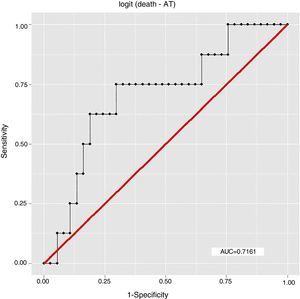
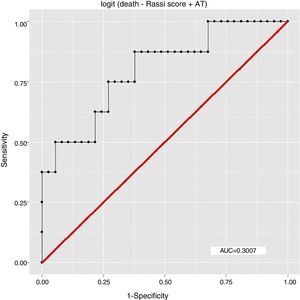
ResultsEight patients (17.78%) had died by September 2015, seven of them (87.5%) from cardiovascular causes, of whom four (57.14%) were considered high risk by the Rassi score. With the Rassi score as independent variable and death as the outcome, we obtained an area under the curve (AUC) of 0.711, with R2=0.214. With AT as independent variable, AUC was 0.706, with R2=0.078. When both Rassi score and AT were defined as independent variables, AUC was 0.800, with R2=0.263.
ConclusionWhen AT is included in logistic regression, it increases the accuracy of the Rassi score for mortality prediction by 5%.
O limiar anaeróbico (LA) é reconhecido como medida objetiva que reflete variações no metabolismo dos músculos esqueléticos no exercício. Seu valor prognóstico nas cardiopatias não chagásicas está bem estabelecido. Entretanto, a avaliação de risco de morte em cardiopatas chagásicos está relativamente estabelecida pelo escore de Rassi. Porém, o valor adicional que o LA pode trazer ao escore não foi estudado ainda.
ObjetivoAvaliar se o LA apresenta um efeito adicional ao escore de Rassi em cardiopatas chagásicos.
MétodosEstudo prospectivo de coorte dinâmica com análise retrospectiva de prontuários, foram analisados 150 prontuários de pacientes. Foram selecionados para a coorte 45 prontuários de pacientes que fizeram teste cardiopulmonar de exercício (TCPE) entre 1996 e 1997 e foram acompanhados até setembro de 2015. Análise dos dados para detectar associação entre variáveis estudadas pode ser vista com um modelo de regressão logística. A adequabilidade dos modelos foi verificada com curvas ROC e o coeficiente de determinação R2.
ResultadosOito pacientes (17,78%) morreram até setembro de 2015, sete (87,5%) por causas cardiovasculares, dos quais quatro (57,14%) eram de alto risco pelo escore. Com escore de Rassi como variável independente, óbito era o desfecho, obtivemos área sob a curva (AUC)=0,711, com R2=0,214. Com LA como variável independente, verificamos AUC=0,706, com R2=0,078. Com a definição do escore de Rassi mais o LA como variáveis independentes, foi obtida AUC=0,800 e R2=0,263.
ConclusãoQuando a variável LA é incluída na regressão logística, ela aumenta em 5% a explicação (R2) à estimativa de morte.
Despite significant reductions in transmission, Chagas disease is still of considerable epidemiological importance in Brazil, due to the large number of infected individuals who may go on to develop severe forms of the disease. The number affected in Brazil is estimated at over a million, one-third of whom have cardiac involvement,1 which is the most serious clinical manifestation, since up to 10% of these patients develop heart failure.2 Mortality from Chagas disease in Brazil remains high and is strongly correlated with the presence of cardiac involvement.2,3
In 2006, Rassi et al.4 published a score for predicting mortality in patients with Chagas cardiomyopathy (CCM). Briefly, of a range of demographic and clinical parameters and non-invasive studies, six were found to be independent predictors of mortality and were used in the score: New York Heart Association class III or IV (5 points), evidence of cardiomegaly (5 points), left ventricular (LV) segmental or global wall-motion abnormality on two-dimensional echocardiography (3 points), non-sustained ventricular tachycardia on 24-hour Holter monitoring (3 points), low QRS voltage (2 points), and male gender (2 points). Patients were classified as low-risk (0-6 points), intermediate-risk (7-11 points), or high-risk (12-20 points).
In individuals with LV systolic dysfunction, systolic volume and cardiac output during exercise are reduced, and there is thus inadequate oxygen (O2) supply to the peripheral muscles. This hampers removal of lactate produced during exertion, lowering blood pH and resulting in metabolic acidosis,5,6 which in turn affects muscle contraction. This is experienced as muscle fatigue7 and hence exercise intolerance.
The anaerobic threshold (AT) is an objective and direct measurement that reflects variations in metabolism of skeletal muscles during exercise.8,9 It has been shown that in patients in the early stages of CCM, AT is already altered, and is higher than in those in advanced stages of the disease, in whom AT decreases in parallel with progressive loss in cardiac and hemodynamic performance.10,11
The prognostic value of AT in non-Chagas heart disease is well established, conferring additional risk to that indicated by other risk factors.8–11 Risk of mortality in CCM is relatively well assessed by the Rassi score.3,4,12,13 However, the added value that AT can bring to the Rassi score has not been studied. Recent developments in pharmacological and non-pharmacological treatment have made this information essential, both for assessment of risk for cardiac death and for decisions concerning treatment and follow-up.
Our aim was thus to assess whether AT presents additional prognostic value to the Rassi score in patients with chronic CCM.
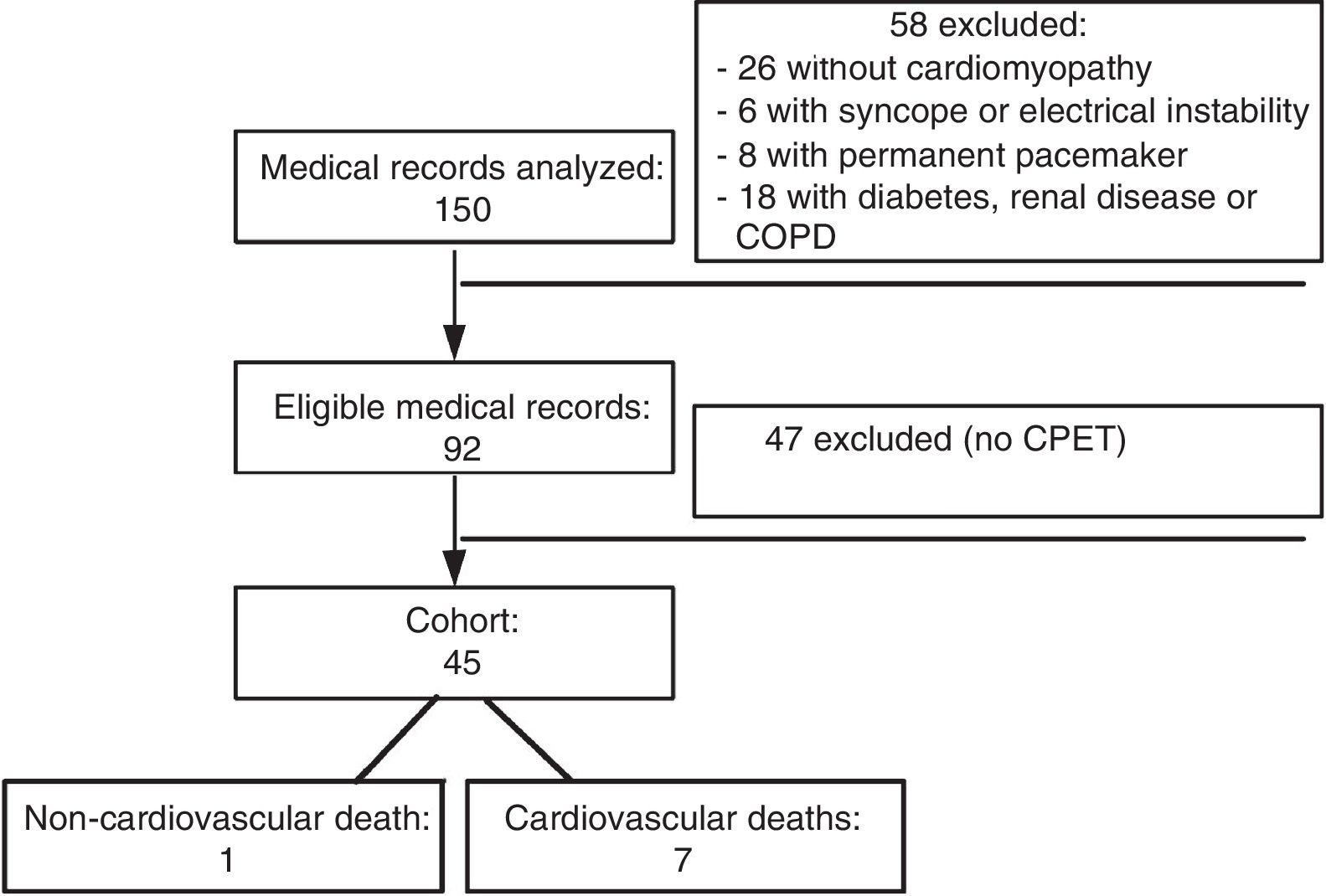
MethodsWe analyzed 150 medical records of patients with evidence of cardiomyopathy from the dynamic cohort of outpatients with CCM at Clementino Fraga Filho University Hospital, Rio de Janeiro, between 1992 and the end of 1999, who underwent cardiopulmonary exercise testing and were followed until September 2015.
The medical records included were of patients with positive serology for Chagas disease by two different testing methods that were positive for Trypanosoma cruzi (indirect immunofluorescence and enzyme-linked immunosorbent assay), who had been away from the endemic area for over 20 years, were aged over 20 years, were under active and regular follow-up, showed evidence of heart disease, had undergone 12-lead resting electrocardiography (ECG), one- and two-dimensional Doppler echocardiography and 24-hour Holter monitoring with two readings up to six months apart, and had performed cardiopulmonary exercise testing (CPET).
Exclusion criteria were: incomplete initial admission data; failure to reach AT on CPET; previous treatment for Chagas infection; permanent pacemaker; atrial and/or ventricular electrical instability with hemodynamic repercussions; clinical or laboratory findings indicative of acute or chronic renal disease, liver disease, refractory heart failure, or thyroid dysfunction at any stage; previous history of chronic obstructive pulmonary disease or type 2 diabetes taking insulin; chronic anemia, smoking, morbid obesity or chronic alcoholism (based on the CAGE questionnaire); signs, symptoms or clinical history of ischemic cardiomyopathy, subsequently confirmed; diagnosis of any other cardiomyopathy; Parkinson disease, neuropathy, or locomotor disorders; and pregnancy or breastfeeding.
On entry into the cohort, all patients underwent complete clinical assessment; laboratory testing (complete blood panel, thyroid function, biochemical analysis, urea, creatinine, blood glucose, lipid panel, liver function, urine sediment, fecal test for parasites); ECG according to the criteria of the 2005 Brazilian Consensus on Chagas Disease14; 24-hour Holter monitoring with two readings at different times by the same observer; and echocardiographic assessment as recommended by the American Heart Association.15
CPET was performed between January 1996 and December 1997 on an electromagnetically braked cycle ergometer (Funbec). Medication (digoxin, diuretics, carvedilol, vasodilators and antiarrhythmics) was suspended 48 hours before CPET and all patients had been in stable clinical condition for at least three months.
A continuous protocol was used with 15-W/min increments and cadence of 60 rpm. The exercise period was preceded by a 4-min resting period and 2-min warm-up without added resistance, and followed by a 12-min recovery period (3 min active at 25 w and 9 min passive).
Concentrations of inspired and expired gases in each respiratory cycle were measured with a gas analyzer (Airspec MGA 2000 mass spectrometer) positioned next to the mouthpiece. The apparatus was calibrated before each test by linear adjustment with two known gas mixtures. Flow was measured with a pneumotachometer (Fleisch #3) warmed to 36°C combined with a differential pressure transducer (Honeywell Microswitch 163PC01D36).
Patients were monitored using a three-channel electrocardiograph (Dixtal SDM 2000) coupled to an oscilloscope. The rhythm recorded by lead DI was initially obtained for 15 min with the patient at rest, then rhythm from lead DI and the continuous ECG trace from leads V1, aVF and CM5 were recorded with the patient exercising.
Only CPET results in which patients reached or exceeded the AT were analyzed. Testing was halted before AT was reached in the event of symptoms that prevented continuation of the test and/or represented a risk to the patient (sustained ventricular tachycardia, conduction disturbances, or bradyarrhythmia).
AT was expressed in relation to O2 consumption (VO2) in ml/kg/min (body temperature and pressure saturated) and was defined as the time when the ventilatory equivalent for oxygen (Ve/VO2) began to increase without a concomitant increase in ventilatory equivalent for carbon dioxide (Ve/VCO2)8,9 and was confirmed by the value of VO2 at which the respiratory exchange ratio (VCO2/VO2) was ≥1 and continued to rise in subsequent respiratory cycles.8,9,16 AT was established by two observers; any disagreement was resolved by consensus.
The study was approved by the hospital's research ethics committee. Since it was a retrospective analysis of medical records, written informed consent was not required.
Statistical analysisA database containing all the study variables was constructed in Excel. R software, version 2.13, was used for the statistical analysis. In descriptive analysis, categorical variables were expressed as frequencies (percentages) and continuous variables as means and standard deviation for normal distributions and median and interquartile range (25%-75%) for non-normal distributions. The data were analyzed using a logistic regression model to detect associations between the study variables. The fit of the models was confirmed using receiver operating curves and the coefficient of determination R2.
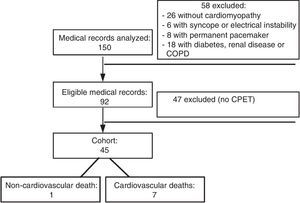
ResultsMedical records of a total of 150 patients with CCM and followed at Clementino Fraga Filho University Hospital up to December 1997 were analyzed. Figure 1 shows the flowchart of analysis of medical records at each stage of the study.
Analysis of the records showed that six patients (13.33%) of the 45 in the cohort were high-risk according to the Rassi score, while the other 39 were low-risk. Eight patients (17.78%) had died by September 2015, seven of them (87.5% of deaths) from cardiovascular causes, four of whom had been classified as high-risk.
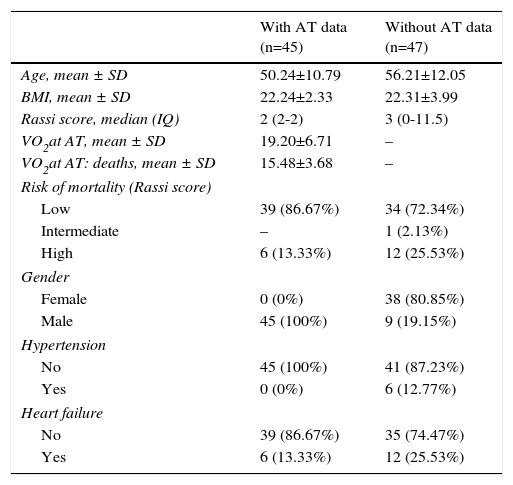
Table 1 presents the demographic and clinical characteristics of the study population (with AT data) and of the 47 patients from the same CCM clinic who were excluded because they did not undergo CPET (no AT data). Those without AT data were slightly older, few were hypertensive and most were female; other characteristics were similar.
Demographic and clinical characteristics of the study population (with aerobic threshold data) and of Chagas cardiomyopathy patients without aerobic threshold data.
| With AT data (n=45) | Without AT data (n=47) | |
|---|---|---|
| Age, mean ± SD | 50.24±10.79 | 56.21±12.05 |
| BMI, mean ± SD | 22.24±2.33 | 22.31±3.99 |
| Rassi score, median (IQ) | 2 (2-2) | 3 (0-11.5) |
| VO2at AT, mean ± SD | 19.20±6.71 | – |
| VO2at AT: deaths, mean ± SD | 15.48±3.68 | – |
| Risk of mortality (Rassi score) | ||
| Low | 39 (86.67%) | 34 (72.34%) |
| Intermediate | – | 1 (2.13%) |
| High | 6 (13.33%) | 12 (25.53%) |
| Gender | ||
| Female | 0 (0%) | 38 (80.85%) |
| Male | 45 (100%) | 9 (19.15%) |
| Hypertension | ||
| No | 45 (100%) | 41 (87.23%) |
| Yes | 0 (0%) | 6 (12.77%) |
| Heart failure | ||
| No | 39 (86.67%) | 35 (74.47%) |
| Yes | 6 (13.33%) | 12 (25.53%) |
AT: anaerobic threshold; BMI: body mass index (kg/m2); IQ: interquartile range; SD: standard deviation; VO2: oxygen consumption (in ml/kg/min).
The association between Rassi score and AT was obtained by a logistic regression model using receiver operating curves with death as the outcome. The results were as follows:
DiscussionThe mean age of patients in the present study was 50.24±10.79 years, close to that found by Viotti et al.17 (46.8 years). In older studies patients were predominantly children, adolescents and young adults; the mean age in Puigbó et al.,18 Moleiro et al.19 and Macedo,20 studies carried out in the 1960s and 1970s, was ≤25 years. This increase in the mean age of Chagas patients reflects successes in reducing vectorial transmission.21 Correspondingly, since the 1990s studies have tended to include adults aged 40 years and older, in view of the natural history of Chagas disease, characterized by slow progression, more advanced cardiomyopathy is to be expected with increasing age. However, in the present study and other urban series4,17 the older mean age means that in most cases the time of this progression may have passed, which may explain why the prevalence of cardiac involvement was lower (86.67% of patients were classified by the Rassi score as low-risk).
The differences between the study population and patients who did not undergo CPET included a greater proportion of females in the latter (there were no females in the study group). Previous studies3,12,13,22 indicate that gender differences do not have clinical implications in these patients. There were few hypertensive patients in the group who did not undergo CPET, and none in the study group; the few who did have hypertension did not present LV hypertrophy on 2D echocardiography, which implies that they did not have significant clinical cardiovascular impairment.22
In 2006, Rassi et al.4 showed that 10-year mortality in patients with CCM and preserved or minimally impaired LV ejection fraction was 10%, a similar figure to all-cause mortality in Brazil.23 However, recent Brazilian registries indicate that life expectancy for patients with CCM, irrespective of severity, is generally improving over time.24 Even so, the epidemiological situation with CCM remains a concern,1 considering that around a million people have CCM in Brazil1 and that, according to Rassi et al.4 the annual overall mortality in outpatients with CCM is 24/1000 patient-years.
Against this background, Rassi et al.4 developed a score to predict overall mortality in patients with CCM that has been amply validated in the literature.3,4,12,13 The Rassi score mainly provides information on LV function, and determination through CPET of functional capacity (which is directly related to the onset of fatigue and exercise intolerance5,6,8,9) gives precise information on oxygen transport and use, i.e. the functional capacity of the lungs and the cardiovascular, muscle and metabolic systems in combination.5 However, since the score does not include variables that objectively assess patients’ exercise capacity, it is reasonable to assume that AT, a variable that provides important information on the limits to an individual's exercise capacity, would present additional prognostic value to the score's prediction model for overall mortality.
In this study, the logistic regression model including both the Rassi score and AT gave an AUC of 0.800, with R2=0.263, while the model including only the Rassi score gave an AUC of 0.71, with R2=0.21. These figures indicate a better fit in models that include AT. Furthermore, besides showing that AT adds prognostic value to the Rassi score for mortality prediction, our results also enable the percentage of this additional value to be calculated as 5%. This is particularly useful given that this information was derived from a cohort made up mainly of patients with less severe cardiac involvement, a population that now accounts for around 70% of patients with CCM.
The reason that AT provides information on a group of mainly low-risk patients is related to the natural history of cardiac involvement in Chagas disease. First affected is the right ventricle, followed by LV diastolic function and then LV systolic function.22 Previous studies25,26 have shown that LV diastolic dysfunction is associated with exercise intolerance. Similarly, right ventricular (RV) function is the major determinant of exercise intolerance in other cardiomyopathies.27
The mechanism by which RV dysfunction affects exercise tolerance appears to be related to elevation of mean pulmonary artery pressure during exertion. Since the right ventricle is highly sensitive to pressure overload, an increase in mean pulmonary artery pressure would increase pulmonary resistance and thus affect RV systolic function. Exercise tolerance is related to mean pulmonary artery pressure at rest.28
Chagas disease can lead to chronic secondary alterations in exercise capacity, in addition to affecting the cardiovascular system, that cause patients discomfort.29 This inability to perform normal physical activity is seen to varying degrees and may be associated with other functional or clinical disorders including impairment of cardiovascular reflexes, lack of cardiovascular adaptation to different functional circumstances, disruption of physiological processes, myocardial contractile dysfunction, regulatory hormone dysfunction, cardiac electrical dysfunction leading to arrhythmias, sudden death, postural hypotension, syncope, neurocirculatory asthenia, coronary syndromes and many other cardiovascular disorders.29
Nevertheless, the reason for assessing exercise capacity is because it provides useful information in a variety of clinical conditions that may cause cardiovascular manifestations. The information obtained may also have therapeutic implications and be an independent prognostic factor, predicting cardiovascular morbidity and mortality.7 Knowledge of patients’ exercise capacity thus goes well beyond its functional and clinical significance.
Studies indicate10,11,30 that exercise capacity is compromised from the earliest stages of Chagas disease as well as in the different stages of cardiac involvement and in heart failure compared to healthy individuals.
The benefits of regular exercise for patients with heart disease have been demonstrated,31,32 leading to acute and chronic metabolic, cardiovascular and ventilatory adaptations in response to increased physiological demands,33 as well as improving exercise capacity due to central and peripheral responses. Recent studies34,35 have demonstrated significant improvements in physical conditioning, exercise capacity and quality of life in patients with CCM following simple and risk-free interventions involving aerobic exercise. However, there are few data in the literature on the benefits of exercise in patients with CCM, and there is a need for further research in this area.36
Some authors37 consider exercise capacity to be a strong predictor of mortality in individuals with and without heart disease, even more powerful than other established risk factors. They also suggest that even small improvements in aerobic conditioning benefit not only exercise capacity but life expectancy.
In view of the well-defined role of CPET in the prognostic assessment of patients with ventricular dysfunction,38 including those with CCM, and in light of our demonstration that AT increases the accuracy of the Rassi score for mortality prediction by 5%, we consider the inclusion of AT in studies assessing prognosis and predicting mortality in CCM to be of considerable interest, since it provides valuable information on patients’ health.
LimitationsThe study design and methods of data analysis do not permit us to determine whether adding the new information obtained from the interaction between AT and the Rassi score changes the mortality risk group.
The study population did not include any patients with intermediate risk and only six (13.33%) with high risk according to the Rassi score. Our conclusions therefore mainly reflect the added value that AT brings to estimating risk of mortality in low-risk patients.
Clinical implicationsExercise intolerance reflecting ventricular dysfunction is an important clinical manifestation in CCM patients, since it can lead to a series of events that can end in death. Different levels of exercise intolerance generally indicate the degree of myocardial impairment,10,11,30 but beyond this, little is known about the possible role of AT in Chagas disease and its relationship with prognosis. Anaerobic respiration has important modulating effects on all the physiological properties of the heart, and changes in these properties are behind certain major cardiac events.5,6,8,9
We set out to determine the additional value of AT for the Rassi score in patients with CCM. The observation that AT brings more information to the Rassi score's prediction of risk of mortality is important, indicating as it does that exercise intolerance as indicated by changes in AT is a prognostic marker at all stages of cardiac involvement in CCM, but further studies are required to confirm this finding. Measures to increase exercise tolerance can be expected to improve survival in patients with CCM.
This study shows that ventricular remodeling is not the only important prognostic factor in CCM. Its main results may make significant contributions to clinical practice in the management of CCM patients, but further studies are needed, with larger patient populations, to support its findings.
ConclusionWhen AT is included as a variable in logistic regression analysis, it increases the coefficient of determination (R2) of the model for prediction of mortality, and thereby increases the accuracy of the Rassi score for mortality prediction in CCM by 5%.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Silva RR, Reis MS, Pereira BB, et al. Valor adicional do limiar anaeróbio em um modelo de predição de morte geral em uma coorte urbana de pacientes com cardiopatia chagásica. Rev Port Cardiol. 2017;36:927–934.
This article is based on the Master's dissertation of the lead author.