Atrial fibrillation (AF) is a complex disease with multiple mechanisms, including the interaction with the autonomic nervous system (ANS), electrophysiological properties of the atria and pulmonary veins (PVs), and vulnerability for AF. We assessed the effects of acute vagal (vagus_stim) and sympathetic stimulation (symp_stim) on atrial conduction, atrial and PV refractoriness and inducibility of AF in an in vivo rabbit model with preserved autonomic innervation.
MethodsAn open-epicardial approach was used in 17 anesthetized and artificially ventilated New Zealand white rabbits. The ECG was recorded with bipolar subcutaneous electrodes placed in the four limbs. Electrograms were obtained with four monopolar electrodes placed epicardially along the atria, and an electrode adapted to the proximal PVs. The cervical vagus nerve and thoracic sympathetic trunk were stimulated with bipolar electrodes. Epicardial activation was recorded in sinus rhythm, and effective refractory periods (ERPs) and conduction times from the high-lateral right atrium (RA) to the high-lateral left atrium (LA) and PVs were quantified at baseline and during vagus_stim, symp_stim, or combined vagal and sympathetic stimulation (dual_stim). Burst pacing (50Hz, 10s), alone or combined with vagus_stim, symp_stim or dual_stim, was performed in the right (RAA) and left atrial appendage (LAA) and PVs to test for AF inducibility.
ResultsAt baseline, ERPs were higher in the LAA and there was a delay in the conduction time from RA to PV, compared to the mean activation time from RA to LA. During vagus_stim or dual_stim, ERP decreased significantly at all sites, and baseline interatrial activation times changed from 20±4ms to 30±10ms and from 21±5ms to 31±11ms, respectively (p<0.05). Symp_stim resulted in a significant decrease in ERPs only in the LAA, and a reduction of the interatrial interval to 16±11ms (p<0.05 vs. baseline). AF inducibility ranged from 35% to 53% with baseline 50Hz pacing, 65–76% during vagus_stim or symp_stim, and 75–100% with dual_stim (p<0.05). AF duration increased significantly during ANS stimulation. In two-thirds of the animals with longer inducible AF, the arrhythmia ceased immediately after cessation of vagus_stim.
ConclusionsIn the fully innervated rabbit heart in vivo, acute ANS stimulation shortens atrial and PV refractoriness, and significantly changes atrial conduction times, promoting AF induction and prolonging the arrhythmia. This underscores the importance of acute variations in ANS tone and its interactions in the pathophysiology of AF.
A influência do sistema nervoso autónomo (SNA) na génese da fibrilhação auricular (FA) envolve múltiplos mecanismos complexos com impacto nas propriedades electrofisiológicas cardíacas. A importância dos efeitos da estimulação autonómica no substrato eléctrico auricular e das veias pulmonares (VP) e na vulnerabilidade para FA requer melhor compreensão.
ObjectivoAvaliar os efeitos da estimulação vagal (estim_vag) e simpática (estim_simp) aguda na condução e refractariedade das aurículas e VP e na inducibilidade de FA no coração de coelho in vivo com inervação autonómica preservada.
MétodosEstudámos 17 coelhos New Zealand de ambos os sexos. Para abordagem de “toráx-aberto” procedeu-se a anestesia, entubação e ventilação após bloqueio neuro-muscular. O ECG foi obtido a partir de 3 derivações dos membros. Os electrogramas foram registados com 4 eléctrodos monopolares colocados na superfície epicárdica, distribuídos ao longo das aurículas e com um eléctrodo circular adaptado à porção proximal das VP. Estimulou-se o nervo vago cervical direito e o tronco simpático torácico com eléctrodos bipolares de platina. Estudámos os períodos refractários efectivos (PRE) e a condução eléctrica auricular, entre a aurícula direita lateral-alta (AD) e a aurícula esquerda lateral-alta (AE), e entre AD e VP, em condições basais e durante estim_vag, estim_simp e estimulação autonómica combinada (dual_estim). Para indução de FA, procedeu-se a pacing rápido (50Hz, 10s, isolado ou com estim_vag, estim_simp ou dual_estim) com eléctrodo bipolar no apêndice auricular direito (AAD), apêndice auricular esquerdo (AAE) e VP.
ResultadosEm condições basais: os PRE eram maiores no AAE e registou-se um atraso na activação da AD para as VP, comparando com a condução interauricular. Durante estim_vag ou dual_estim: os PRE encurtaram significativamente em todos os locais, o intervalo de condução interauricular variou de 20±4ms para 30±10ms (p<0,05) e 31±11ms (p<0,05), respectivamente. Com estim_simp obteve-se uma redução significativa dos PRE no AAE e do tempo de condução interauricular para 16±11ms (p<0,05). Induziu-se FA em 35% a 53% dos animais com 50Hz, 65% a 76% com estim_vagal ou estim_simp, e 75% a 100% com dual_estim (p<0,05). A duração da FA aumentou significativamente durante estim_vagal e/ou estim_simp. Em 2/3 dos animais com indução de FA com duração >10s a arritmia terminou imediatamente após interrupção da estim_vagal.
ConclusõesNo coração de coelho inervado in vivo, a estimulação autonómica aguda encurta a refractariedade auricular e das VP, e modifica a velocidade de condução auricular, potenciando a indução e duração de FA. Os resultados sugerem que as variações agudas e a interacção da actividade autonómica podem desempenhar um papel importante na fisiopatologia da FA.
Atrial fibrillation (AF) is a complex arrhythmia involving multiple mechanisms that are not fully understood despite recent advances in its treatment. Lone AF is probably caused by an electrophysiological substrate involving focal automaticity arising mainly from the pulmonary veins (PVs), together with multiple depolarization waves that propagate and interact over the atrial surface.1–4 It has been suggested that the activity of the autonomic nervous system (ANS) influences the electrophysiological properties of the atria and thus modulates the pathophysiology of AF.4–6 Autonomic tone can promote reentry arrhythmias and triggered activity.4,5,7 Clinical studies suggest that sympathetic and parasympathetic innervation may be important in AF genesis, and there is evidence that episodes of paroxysmal AF are often preceded by autonomic tone variations.8–10 Experimental studies have also improved our understanding of the arrhythmogenic effects of the ANS on the mechanisms underlying AF.11–16 Using a canine model, it was suggested that atrial arrhythmias were caused by the effects of changes in autonomic activity on the PVs and superior vena cava.13 In a similar model, sympathetic and vagal stimulation were shown to shorten atrial effective refractory periods (ERPs) and to promote reentry.17 The same study also found that heterogeneous distribution of autonomic innervation in the atria may contribute to the substrate associated with AF, particularly in the absence of structural abnormalities.17 It has been demonstrated that stimulation of cardiac ganglionated plexi causes local release of sympathetic and parasympathetic neurotransmitters that increase automaticity and shorten local action potential duration and refractoriness.15 A recent study in a canine model, with direct recording of efferent autonomic activity, demonstrated that simultaneous sympathovagal discharges are common triggers of paroxysmal atrial tachycardia and AF following rapid left atrial pacing.18 While it is recognized that the ANS plays an important role in the pathophysiology of AF, the impact of sympathetic and vagal activity (alone or in combination) on atrial arrhythmogenesis and on the initiation, maintenance and termination of AF has yet to be clarified. Various animal models have been developed to characterize the electrophysiological effects of autonomic stimulation in order to improve understanding of the complex mechanisms underlying AF. However, a large proportion of these experimental studies have been performed in isolated heart models. There is therefore a need to clarify the relationship between acute autonomic stimulation in vivo and vulnerability for initiation and maintenance of AF, since modification of autonomic innervation, particularly in cardiac areas with a high density of nerve endings, could have implications for AF ablation.14,19,20
The present study assessed the effect of acute sympathetic and vagal stimulation on atrial conduction and atrial and PV refractoriness, as well as on AF inducibility and duration, in an in vivo rabbit model with preserved autonomic innervation.
MethodsPreparation of animal modelWhite New Zealand rabbits of both sexes (n=17, 3.0–4.9kg) were anesthetized with sodium pentobarbital (60mg/kg, iv). A cannula was inserted below the larynx by tracheotomy for intermittent ventilation with positive pressure and an air/O2 mixture (model 607 respiration pump, Harvard Apparatus, UK; 60cycles/min) after neuromuscular blockade (vecuronium 0.05mg/kg/h, iv) to control respiratory rate and volume. The level of anesthesia was assessed by monitoring blood pressure (BP), heart rate (HR) and respiratory activity, using supplementary drug doses to maintain stable general anesthesia. The femoral artery was cannulated for continuous BP monitoring using a SensoNor 840 transducer and the femoral vein was used for administration of 0.9% saline solution and the drugs. The bladder was catheterized and drained. Rectal temperature was maintained at ∼38°C by means of a heating pad (Harvard Apparatus, UK) placed under the animal. The ECG was obtained with bipolar subcutaneous electrodes placed in all four limbs and the signal was amplified and filtered. The ECG, BP and electrograms were continuously monitored on a polygraph (Lectromed, Hertfordshire, UK) and analyzed (PowerLab software, ADInstrument).
The anesthetized animal was placed in ventral decubitus to expose the dorsal portion of the thorax, and an incision was made at the level of the C6 and T2 vertebrae, using electrocauterization and an automatic retractor to access the T1 vertebra, which was perforated with a 2mm drill to expose the spinal dura mater. With the animal in dorsal decubitus, a median incision was made in the neck and the right cervical vagus nerve was identified; this was then isolated and prepared for bipolar electrical stimulation with platinum electrodes, isolated with soft paraffin. A slight rotation enabled a bipolar electrode to be inserted through the spinal canal using a micromanipulator (M330, WPI) to stimulate thoracic sympathetic flow at the level of T1, which contains mainly preganglionic fibers to the stellate ganglion.21 The electrodes for vagus nerve and sympathetic trunk stimulation were connected to a multi-channel programmable pulse generator (Master-8, AMPI, Israel). The vagus nerve was stimulated (20Hz, 2-ms impulse), with the amplitude adjusted to obtain a ≈50% decrease in HR, and sympathetic stimulation (3Hz, 1-ms impulse) was tested to obtain increases in HR and BP.
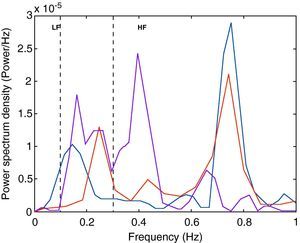
Besides monitoring HR and BP, we recorded 30-s series of RR intervals for spectral analysis using fast Fourier transform and MatLab software (MathWorks, Natick, MA, USA) in Origin (OriginLab, Origin Lab Corporation, Northampton, MA, USA) to confirm autonomic stimulation, in accordance with a previously described method.22,23 The spectral frequency was divided into three components: very low-frequency (VLF: 0.01–0.1Hz), low-frequency (LF: 0.1–0.3Hz) and high-frequency (HF: 0.3–1Hz). The HF band was attributed to vagal modulation and the LF band was considered to reflect sympathetic activity.
A median sternotomy was performed and the pericardium opened to expose the heart and for epicardial electrophysiological study of the atria and PVs. Following thoracotomy, an end-expiratory pressure of 1–2cm H2O was maintained.
The experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health, and were approved by the Ethics Committee of the Faculty of Medicine of Lisbon University.
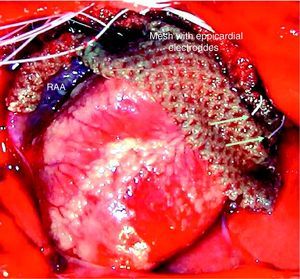
Electrophysiological protocolThe electrograms were recorded with four monopolar electrodes (125-μm Teflon-insulated Ag/AgCl), placed in a nylon mesh with 4-mm spacing and applied in epicardial position, from the high lateral right atrium (RA) to the high lateral left atrium (LA) (Fig. 1). A circular electrode (coaxial cable) was placed around the proximal segment of the left PVs for stimulation and electrogram recording. The electrograms were amplified and filtered from 0.5 to 300Hz and from 30 to 250Hz for monopolar and bipolar recordings, respectively (Neurolog, Digitimer, UK). At the end of the procedure, the animals were sacrificed with an overdose of anesthetic.
Epicardial activation was recorded in sinus rhythm, and conduction times from RA to LA and from RA to PV depolarization were measured in baseline conditions and during vagal, sympathetic or combined sympathovagal stimulation. Stimulation to study ERPs and assess AF inducibility was performed with a bipolar electrode (0.1mm, Teflon-insulated silver), positioned with a micromanipulator (M330, WPI) in the RAA and the LAA, and with a circular electrode in the PVs. In stable conditions, the programmed stimulation protocol was performed (Master-8 stimulator, AMPI, Israel), with an extrastimulus being introduced during continuous bipolar pacing with a 300-ms cycle length (or 20ms less than the RR interval for a heart rate of >200bpm), using 1-ms impulses and twice the amplitude of the capture threshold, and a coupling interval 100ms shorter than the baseline pacing cycle, which was then reduced in 5-ms steps until the ERP was reached. The ERP was taken as the longest S1–S2 interval that failed to initiate local depolarization. Conduction times and ERPs were analyzed in baseline conditions and during vagal, sympathetic and combined stimulation. To assess vulnerability for AF induction, burst pacing (50Hz, 10s, suprathreshold intensity) was used, with the bipolar electrode situated in the RAA, LAA and PVs, alone or combined with vagal, sympathetic or sympathovagal stimulation. The induction protocol was applied up to three times at each stimulation site. AF was defined as a rapid disordered atrial rhythm, with variable cycle length, polarity, configuration and amplitude, and lasting more than five cycles.24 AF duration was assessed under each of the experimental conditions.
Statistical analysisContinuous variables were expressed as means±standard deviation and categorical variables as frequencies and percentages. Comparisons were made using the ANOVA test and the Student's t test for continuous variables. The Kolmogorov–Smirnov test was used to assess the normality of distribution of continuous variables. ERP values and conduction times, in baseline conditions and during autonomic stimulation, were compared by the paired Student's t test. The chi-square test with Yates correction was used for categorical variables. Results with p<0.05 were considered significant. The statistical package used was GraphPad (GraphPad Software Inc., CA, USA).
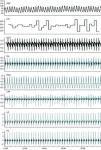
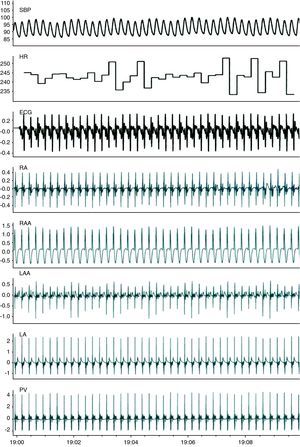
ResultsFig. 2 shows the data recorded in baseline conditions. After preparation of the animal, mean HR was 211±37bpm (190–230bpm) and systolic BP was 80±17mmHg (65–90mmHg). Vagal stimulation significantly reduced HR (from 214±30 to 100±31bpm, p<0.01) and BP (from 79±23 to 58±20mmHg, p<0.01) (Fig. 3 and Table 1). Preganglionic sympathetic stimulation induced a slight increase in HR (from 210±34 to 237±37bpm, p<0.05) and in BP (75±13 to 91±19mmHg, p<0.01) (Fig. 3 and Table 1). After cessation of autonomic stimulation, HR and BP returned to pre-stimulation values.
Effects of autonomic stimulation on heart rate, systolic blood pressure, electrophysiological parameters and atrial fibrillation inducibility.
| Basal | Vagal | Sympathetic | Sympathovagal | |
| HR (bpm) | 211±37 | 100±31b | 237±37a | 190±40a |
| Systolic BP (mmHg) | 80±17 | 58±20b | 91±19b | 70±21 |
| RA-LA conduction (ms) | 20±4 | 30±10a | 16±6a | 31±11a |
| RA-PV conduction (ms) | 24±6 | 36±14a | 17±8b | 29±14 |
| RAA ERP (ms) | 69±16 | 48±17a | 64±15 | 51±20a |
| LAA ERP (ms) | 83±16c | 53±21a,c | 56±23a | 51±23a |
| PV ERP (ms) | 59±16 | 41±17a | 49±19 | 40±18a |
| AF duration (s) | 2.6±1.4 | 6.8±2.5a | 9.6±3.7a | 6.1±1.4a |
Abbreviations as in text.
Fig. 4 shows the spectral analysis of RR intervals and the distribution of frequency bands associated with sympathetic and parasympathetic flow to the heart. Compared to baseline values, vagal stimulation increased HR variability, reducing the LF/HF ratio, and sympathetic stimulation increased the LF component and the LF/HF ratio.
Conduction times between the RA and the LA increased with vagal stimulation from 20±4ms to 30±10ms (p<0.05), and decreased to 16±6ms with sympathetic stimulation (p<0.05). Combined sympathovagal stimulation also significantly changed interatrial conduction (from 21±5ms to 31±11ms, p<0.05). Conduction times between the RA and the PVs increased during vagal stimulation (p<0.05) and decreased with sympathetic stimulation (p<0.01), but did not change significantly with sympathovagal stimulation (Table 1).
With regard to ERP values (Table 1), refractoriness was higher in the LAA than in the RAA and PVs (83±16ms vs. 69±16ms and 59±16ms, respectively; p<0.01) in baseline conditions. Vagal and sympathovagal stimulation consistently reduced ERPs significantly in the RAA, LAA and PVs, while sympathetic stimulation resulted in a decrease in ERPs only in the LAA. During autonomic stimulation, ERP shortening was more marked in the LAA than at the other sites. However, with vagal stimulation alone, the differences in ERPs between the PVs and the LAA remained statistically significant (p<0.05).
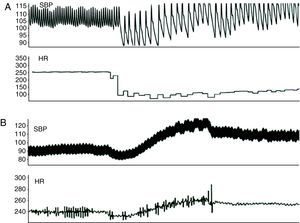
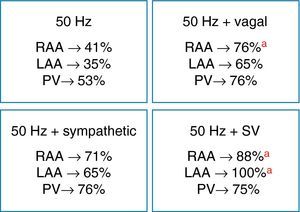
Effect of acute autonomic stimulation on atrial fibrillation inducibilityAutonomic stimulation had a significant effect on the induction and duration of AF (Table 1). Burst pacing at 50Hz in the RAA, LAA and PVs induced AF intermittently in 41%, 35% and 53% of the rabbits, respectively. During vagal stimulation, AF was reproducibly induced in 76%, 65% and 76% of the animals, with burst pacing in the RAA, LAA and PVs, respectively (50Hz vs. 50Hz+vagal stimulation, p<0.05 for the RAA), and AF duration increased significantly with pacing in the RAA (6.8±2.5 vs. 1.8±1.2s, p<0.01) and PVs (3.6±1.6 vs. 1.7±0.7s, p<0.05). In four animals, AF was induced with an extrastimulus combined with vagal stimulation during assessment of ERPs in the RAA.
AF inducibility increased with sympathetic stimulation (71%, 65% and 76% for the RAA, LAA and PVs, respectively) (Fig. 5), without reaching statistical significance compared to burst pacing alone. In two animals, short periods of AF were induced with an extrastimulus at the different sites, and spontaneous premature depolarization originating in the PVs occurred in another two. AF duration increased significantly only with PV stimulation (9.6±3.7 vs. 1.7±0.7s, p<0.05). During combined sympathovagal stimulation, AF induction was reproducible with burst pacing in the RAA, LAA and PVs in 88%, 100% and 75% of the animals, respectively (50Hz vs. 50Hz+sympathovagal stimulation, p<0.05 for the RAA and LAA), and AF duration increased at all sites. AF duration was >10s in 37.5% of the rabbits (only with vagal or sympathovagal stimulation), and the arrhythmia ceased immediately after cessation of vagal stimulation in two-thirds of these cases.
DiscussionThe present study analyzes the effects of acute autonomic stimulation on the electrophysiological properties of the atria and PVs and on vulnerability for AF induction in an in vivo rabbit heart model. Using a consistent methodology, we characterized the effect of direct stimulation of the right cervical vagus nerve and the thoracic sympathetic trunk on electrical conduction and parameters of atrial and PV refractoriness, and demonstrated that ANS activity can contribute to the induction and duration of AF.
Our results indicate that there is a conduction delay in sinus rhythm between the atria and PVs, possibly due to a delay at the LA–PV junction, which appears to be associated with slowed conduction.25 In this experimental model, there was a significant delay in interatrial conduction during vagal stimulation, and a reduction in conduction times during sympathetic stimulation. Mean interatrial conduction time was twice as long during vagal stimulation, with a lesser effect on RA–PV conduction, suggesting that the response to vagal activation is non-uniform and that the impact on electrical conduction delays varies, possibly related to the heterogeneous effect of vagal innervation on atrial conduction properties.26 ERPs in the LAA were significantly longer than those recorded in the RAA and PVs, representing heterogeneous refractoriness at the sites assessed, both in baseline conditions and during vagal stimulation. The latter reduced atrial and PV refractoriness, while sympathetic activation resulted in a significant decrease in ERPs only in the LAA. The variable effects of autonomic modulation on conduction and refractoriness of the atria and PVs may be related to regional differences in cardiac innervation that affect the functional substrate of AF, which is known to depend on changes in conduction and ERP shortening, thus promoting multiple reentrant circuits in atrial tissue.25,27 The shorter ERPs in the PVs and RAA may explain the greater AF inducibility with burst pacing at these sites. The consistent increase in AF induction and duration during acute autonomic stimulation suggests that rapid variations in local conduction and refractoriness and in ANS tone may be determining factors in vulnerability for AF.
Animal models in the study of vulnerability for atrial fibrillationIn recent years, animal experiments have sought to elucidate the basic mechanisms underlying the pathophysiology of AF and have considerably improved our understanding of electrophysiological concepts such as simultaneous multiple depolarization waves with variable spatial orientation, rapid focal activity with fibrillatory conduction, and rotors resulting in the identification of areas of fragmented atrial electrical activity. However, the development of in vivo models with preserved autonomic innervations that enable dynamic electrophysiological assessment with different types of ANS activity, and analysis of their relation to AF induction and duration, remains a challenge.
In dogs, microreentry, automaticity and triggered activity have been suggested as mechanisms associated with atrial arrhythmias.28,29 Hayashi et al. used cervical vagal stimulation to induce AF with atrial pacing bursts in anesthetized dogs.30 In recent studies, vagal stimulation was associated with shortening of atrial ERPs and AF induction in dogs.17,31 These vagal effects and AF inducibility can be modified by epicardial ablation of the ganglionated plexi.12,15 In patients undergoing AF catheter ablation, recurrence of AF is reduced if the procedure includes selective vagal denervation.32,33 The idea that cardiac denervation can play a role in AF prevention is a complex but promising approach that requires further investigation.
In previous studies, sympathetic stimulation has been less effective than vagal stimulation in influencing atrial refractoriness and AF induction.11,34 However, in a canine model using chronic rapid pacing, sympathetic stimulation induced repetitive activity in the PVs and contributed to initiation of AF.35 Combined sympathetic and parasympathetic stimulation can also affect electrophysiological properties and increase vulnerability for AF in dogs.16,35 The electrophysiological effects of autonomic reflex activity in the atria and PVs may thus play an important role in the pathophysiology of AF.
The sequence of mechanisms responsible for spontaneous termination of AF has yet to be clarified. It may be that resolution (or reversal) of the changes in autonomic tone that accompany initiation of AF also contribute to its termination. Tomita et al. demonstrated that the changes in ANS activity that precede initiation of AF episodes normalize immediately after termination of AF.8 In the present study, using an intact rabbit heart model that has little propensity for AF in baseline conditions, we confirmed that sympathovagal interaction has significant effects on conduction and refractoriness of the atria and PVs and contributes to AF inducibility and duration. Furthermore, vagal activity appeared to exert a direct influence on termination of AF, since in two-thirds of the animals with longer AF, the arrhythmia ceased immediately after cessation of vagal stimulation.
Evidence for the role of the ANS in the genesis of paroxysmal AF has been provided by clinical studies and experimental models. Despite conflicting results of analyses of HR variability in the period preceding spontaneous initiation of AF, recurrent paroxysmal episodes appear to be related to dynamic variations in autonomic tone, possibly the result of synergy between adrenergic flow and vagal activity.5,18,36,37 Further studies are required to elucidate the effect of innervation and the interaction of sympathovagal reflex activity on the complex dynamics of the functional substrate of atrial arrhythmogenesis.
Study limitationsAssessment of refractoriness was based on only three sites (RAA, LAA and PVs). The lengthy and detailed process involved in calculating ERPs in baseline conditions and with different autonomic stimulation would have been impractical, particularly in terms of maintaining the stability of the in vivo preparation. However, the technique of programmed electrical stimulation with an extrastimulus has been widely used to study refractoriness. Future studies using different methods may be warranted to confirm these results. Nevertheless, in the present study electrophysiological changes, and their effect on AF induction, occurred immediately after autonomic stimulation, suggesting a clear relationship between vagal and sympathetic modulation and the results obtained.
Finally, although AF induction reflects vulnerability, the difficulty in sustaining the arrhythmia in the small atrial area of the rabbit heart makes it difficult to study the electrophysiological mechanisms underlying sustained AF with this model.
ConclusionsThis experimental in vivo model with preserved innervation enabled assessment of the effects of vagal and sympathetic stimulation on the electrophysiological properties of the atria and PVs. The results show that vagal activity is associated with increased interatrial conduction and shortening of ERPs in the atria and PVs, while sympathetic stimulation increases conduction velocity between the atria and between the atria and the PVs, but reduces refractoriness only in the LAA. This preparation also enables assessment of the effects of autonomic stimulation on the inducibility and duration of AF. Our findings suggest that this experimental model can provide valuable information regarding the influence of autonomic activity and its interactions on the pathophysiological mechanisms of AF.
Ethical responsibilitiesProtection of human and animal subjects: The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Oliveira, M; Modulação electrofisiológica das aurículas e veias pulmonares: interacção da estimulação simpática e parassimpática na inducibilidade de fibrilhação auricular aguda num modelo experimental in vivo. Rev Port Cardiol 2012. doi:10.1016/j.repc.2012.01.007.