Thoracic endovascular aortic repair (TEVAR) is a minimally invasive technique which is increasingly used in different thoracic aortic pathologies such as aortic aneurysm, complicated type B aortic dissection, aortic trauma, intramural hematoma and penetrating aortic ulcer.
In this paper we discuss the main indications for endovascular stent-grafts in the treatment of thoracic aortic disease, based on three cases in which this procedure was used for three different conditions: degenerative aneurysm, complicated type B dissection and post-traumatic injury.
These case reports add to the evidence that TEVAR is a safe and feasible therapeutic alternative in selected patients with thoracic aortic disease, improving aortic remodeling, with relatively low morbidity and mortality. The main complications and difficulties related to the procedure are also discussed.
O tratamento endovascular é utilizado num número cada vez maior de doentes, com diferentes tipos de patologias da aorta torácica. Atualmente, esta opção terapêutica deve ser considerada no tratamento dos aneurismas da aorta torácica, na dissecção aguda da aorta do tipo B complicada, nas lesões pós-traumáticas, nos hematomas intramurais e nas úlceras penetrantes da aorta.
Neste artigo pretendemos discutir as indicações para a utilização desta técnica percutânea, partindo da apresentação de três casos clínicos, tratados no nosso centro, com diferentes patologias da aorta torácica: aneurisma degenerativo, dissecção aguda da aorta do tipo B, complicada, e lesão pós-traumática da aorta.
A discussão destes três casos clínicos ajudará a evidenciar que o tratamento endovascular é uma boa alternativa terapêutica em doentes selecionados com diferentes patologias da aorta torácica, associando-se a taxas de mortalidade relativamente baixas, a reduzida morbilidade e a curtos tempos de hospitalização. Serão ainda abordadas as mais recentes recomendações para a sua utilização, assim como as principais complicações e dificuldades associadas a este procedimento minimamente invasivo.
Thoracic endovascular aortic repair (TEVAR) is a therapeutic option which is increasingly used in different pathologies.1,2 Several studies have demonstrated the advantages of this treatment: a recent meta-analysis showed that patients treated by TEVAR had lower periprocedural mortality and fewer neurological, bleeding, cardiac and respiratory complications compared to surgery.3 Despite these good results, there are still concerns about the efficacy, safety and long-term durability of this new treatment.
In this paper we describe the experience of our interventional cardiology unit in the use of this minimally invasive technique, based on a series of three patients with different thoracic aortic pathologies who were treated by TEVAR.
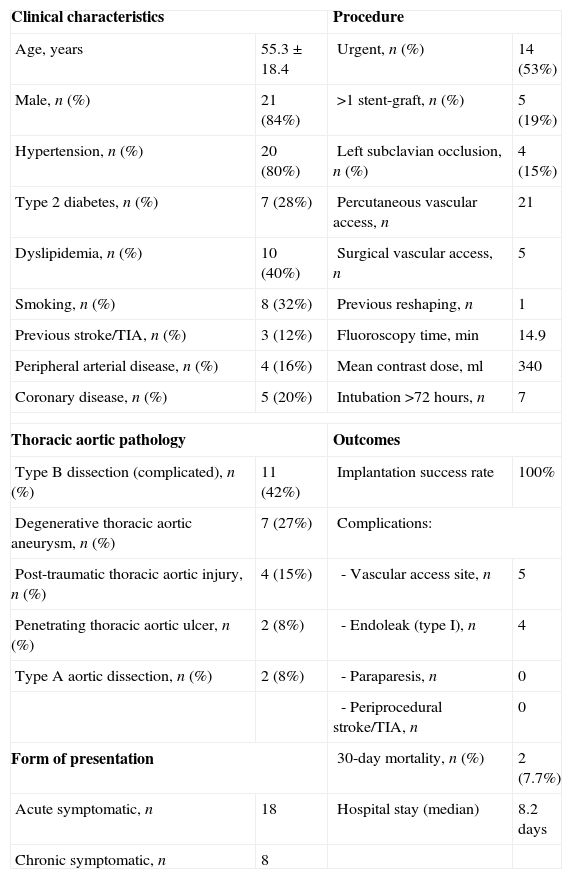
This treatment has now been used in our center in 26 patients with various types of thoracic aortic disease, with very promising results. Table 1 summarizes our center's experience in this technique, including indications, outcomes and complications.4
Population characteristics, procedural data and outcomes for patients treated in our center by TEVAR (n=26).
| Clinical characteristics | Procedure | ||
| Age, years | 55.3±18.4 | Urgent, n (%) | 14 (53%) |
| Male, n (%) | 21 (84%) | >1 stent-graft, n (%) | 5 (19%) |
| Hypertension, n (%) | 20 (80%) | Left subclavian occlusion, n (%) | 4 (15%) |
| Type 2 diabetes, n (%) | 7 (28%) | Percutaneous vascular access, n | 21 |
| Dyslipidemia, n (%) | 10 (40%) | Surgical vascular access, n | 5 |
| Smoking, n (%) | 8 (32%) | Previous reshaping, n | 1 |
| Previous stroke/TIA, n (%) | 3 (12%) | Fluoroscopy time, min | 14.9 |
| Peripheral arterial disease, n (%) | 4 (16%) | Mean contrast dose, ml | 340 |
| Coronary disease, n (%) | 5 (20%) | Intubation >72 hours, n | 7 |
| Thoracic aortic pathology | Outcomes | ||
| Type B dissection (complicated), n (%) | 11 (42%) | Implantation success rate | 100% |
| Degenerative thoracic aortic aneurysm, n (%) | 7 (27%) | Complications: | |
| Post-traumatic thoracic aortic injury, n (%) | 4 (15%) | - Vascular access site, n | 5 |
| Penetrating thoracic aortic ulcer, n (%) | 2 (8%) | - Endoleak (type I), n | 4 |
| Type A aortic dissection, n (%) | 2 (8%) | - Paraparesis, n | 0 |
| - Periprocedural stroke/TIA, n | 0 | ||
| Form of presentation | 30-day mortality, n (%) | 2 (7.7%) | |
| Acute symptomatic, n | 18 | Hospital stay (median) | 8.2 days |
| Chronic symptomatic, n | 8 | ||
TIA: transient ischemic accident.
A 50-year-old man with several cardiovascular (CV) risk factors (hypertension, dyslipidemia and smoking) had been involved in a car accident at the age of 14, with no apparent complications at the time.
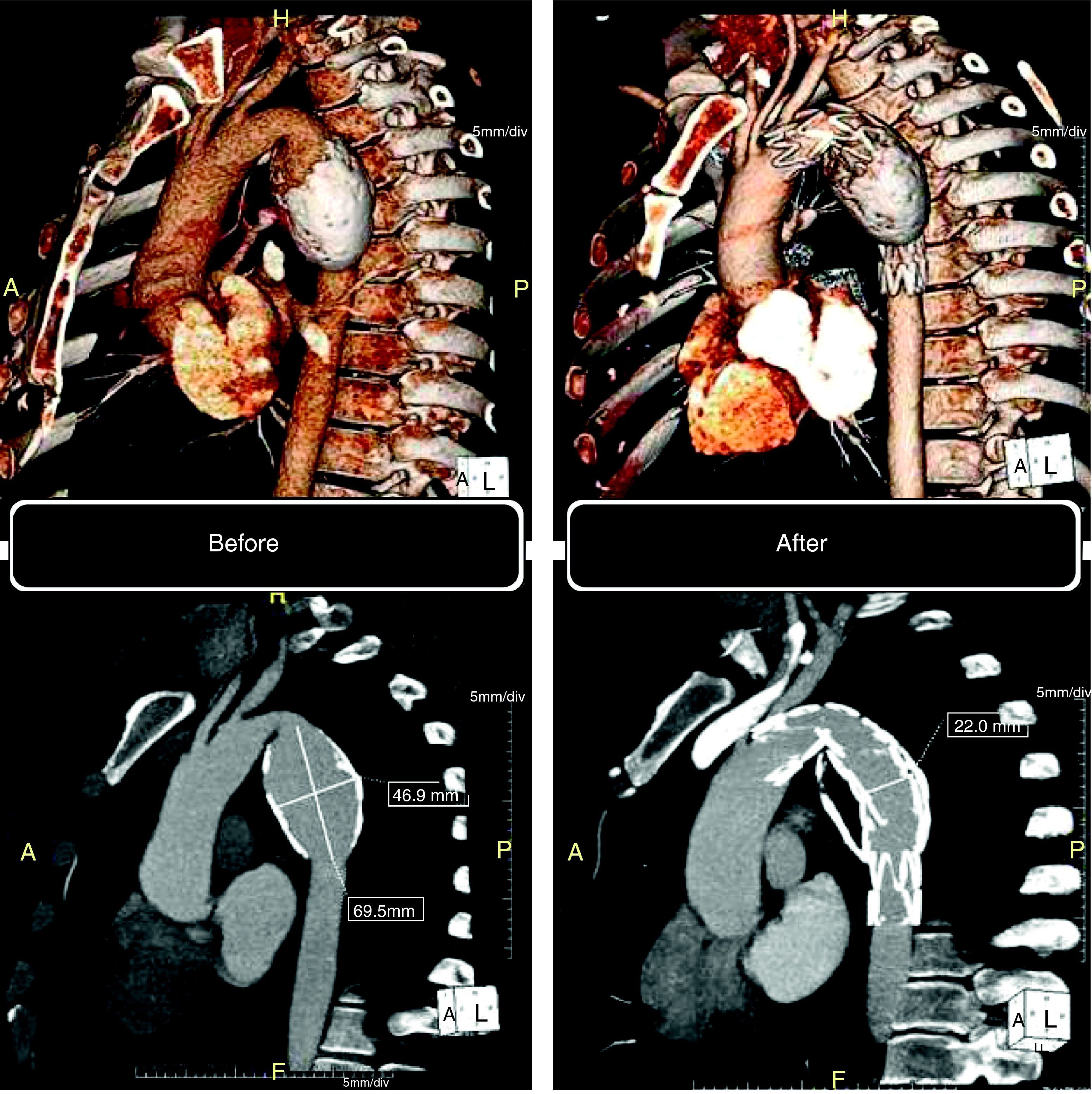
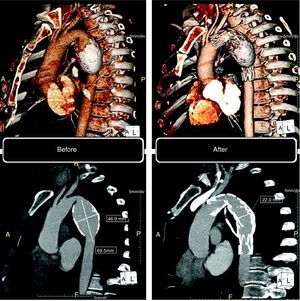
This patient had been followed by his general practitioner since 2005 for an asymptomatic fusiform aneurysm of the descending thoracic aorta, which had been discovered by accident. In January 2010 he was referred to the cardiothoracic surgery unit of our hospital due to dorsal thoracic pain of short duration and not associated with exertion. A 64-slice cardiac CT scan (Somatom Sensation 64, Siemens, Erlangen, Germany) revealed an aneurysm of the descending thoracic aorta measuring 46.9mm×55.2mm×69.5mm, with circumferential atheromatous calcification (Fig. 1). Following discussions between the thoracic surgeon and the interventional cardiologist, and since after receiving detailed information the patient refused surgery, it was decided to adopt a percutaneous approach to exclude the aneurysm.
Computed tomography: volume-rendering technique (VRT) (top) and maximum intensity projection (MIP) (bottom). Left: before the procedure, aneurysm of the descending thoracic aorta, with circumferential atheromatous calcification. Right: six months after the procedure, confirming complete exclusion of the aneurysm.
In June 2010 a 26mm×150mm endovascular stent-graft (Valiant Captivia®, Medtronic) was implanted under general anesthesia using a right femoral access, subsequently closed with a Perclose® ProGlide closure device (Abbott Vascular). The patient's clinical course was uneventful and he was discharged seven days after the procedure.
Control CT angiography six months after the procedure showed complete exclusion of the aneurysm (Fig. 1). The patient is asymptomatic, has quit smoking and his CV risk factors are under control with statins, beta-blockers and ACE inhibitors.
Case B: acute type B aortic dissectionAn 84-year-old man with CV risk factors of hypertension, diabetes and obesity (body mass index 30.1kg/m2) but no CV or other relevant history, went to the emergency department in April 2008 with retrosternal chest pain radiating to the back of about six hours’ evolution. After acute myocardial ischemia was excluded he was diagnosed by CT angiography with acute type B dissection of the thoracic aorta complicated by mediastinal hematoma, moderate bilateral hemothorax and new-onset anemia (fall in hemoglobin from 14.4 to 8.9g/dl).
After discussion with the cardiothoracic surgery team, the patient was rejected for surgery and conservative medical therapy was begun, but there was no significant clinical improvement.
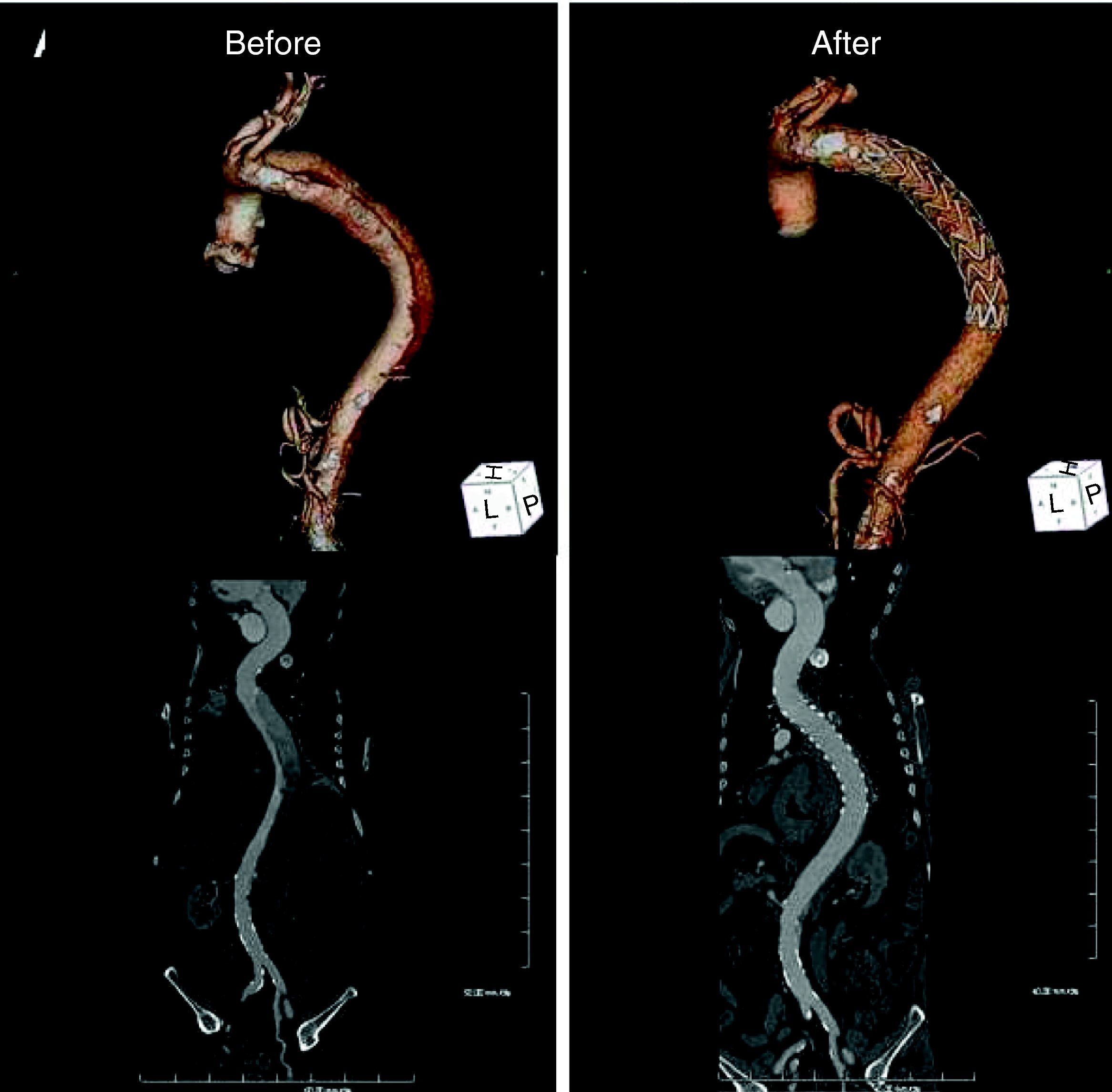
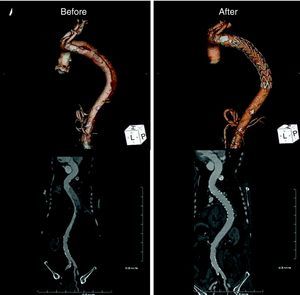
Five days after symptom onset he underwent 64-slice CT angiography, which revealed dissection of the descending thoracic aorta, its entry tear 5cm distal to the emergence of the left subclavian artery (maximum aortic diameter 42mm×49mm; true lumen of 19mm and false lumen of 27mm), extending to the renal arteries (maximum extension of 409mm), the false lumen involving the origin of the left renal artery (Fig. 2).
Computed tomography: volume-rendering technique (VRT) (top) and curved multiplanar reconstruction (bottom). Left: before the procedure, dissection of the descending thoracic aorta extending to the renal arteries and involving the origin of the left renal artery. Right: six months after the procedure, showing the endovascular stent-graft covering the entry tear, disappearance of the false lumen and normalization of renal artery perfusion.
As the patient was still symptomatic, it was decided to proceed with endovascular treatment, a 30mm×200mm endovascular stent-graft (Valiant®, Medtronic) being implanted percutaneously under general anesthesia using a right femoral approach and a Perclose® ProGlide closure device (Abbott Vascular). The postoperative course included nosocomial pneumonia, which responded well to antibiotics, and acute renal failure. The patient was discharged home, asymptomatic, 12 days after the procedure.
Follow-up CT six months after the intervention showed the endovascular stent-graft covering the entry tear, disappearance of the false lumen and dissection, and normalization of renal artery perfusion (Fig. 2, right).
After nearly three years of follow-up, the patient has no CV symptoms. In June 2009, following acute cholecystitis, he underwent laparoscopic cholecystectomy, without complications.
Case C: acute post-traumatic thoracic aortic injuryA 21-year-old man, a smoker, with no CV or other relevant history, was involved in a head-on car accident in April 2007. On admission to the district hospital he presented anterior chest pain radiating to the back that was not easily controlled by analgesics. He also had right hip dislocation that was reduced in the orthopedic department.
As his chest pain persisted he was transferred to our center. On admission he was hemodynamically and electrically stable with no alterations on the neurological exam, but still symptomatic. Laboratory tests revealed anemia (hemoglobin 11.0g/dl), apparently of new onset.
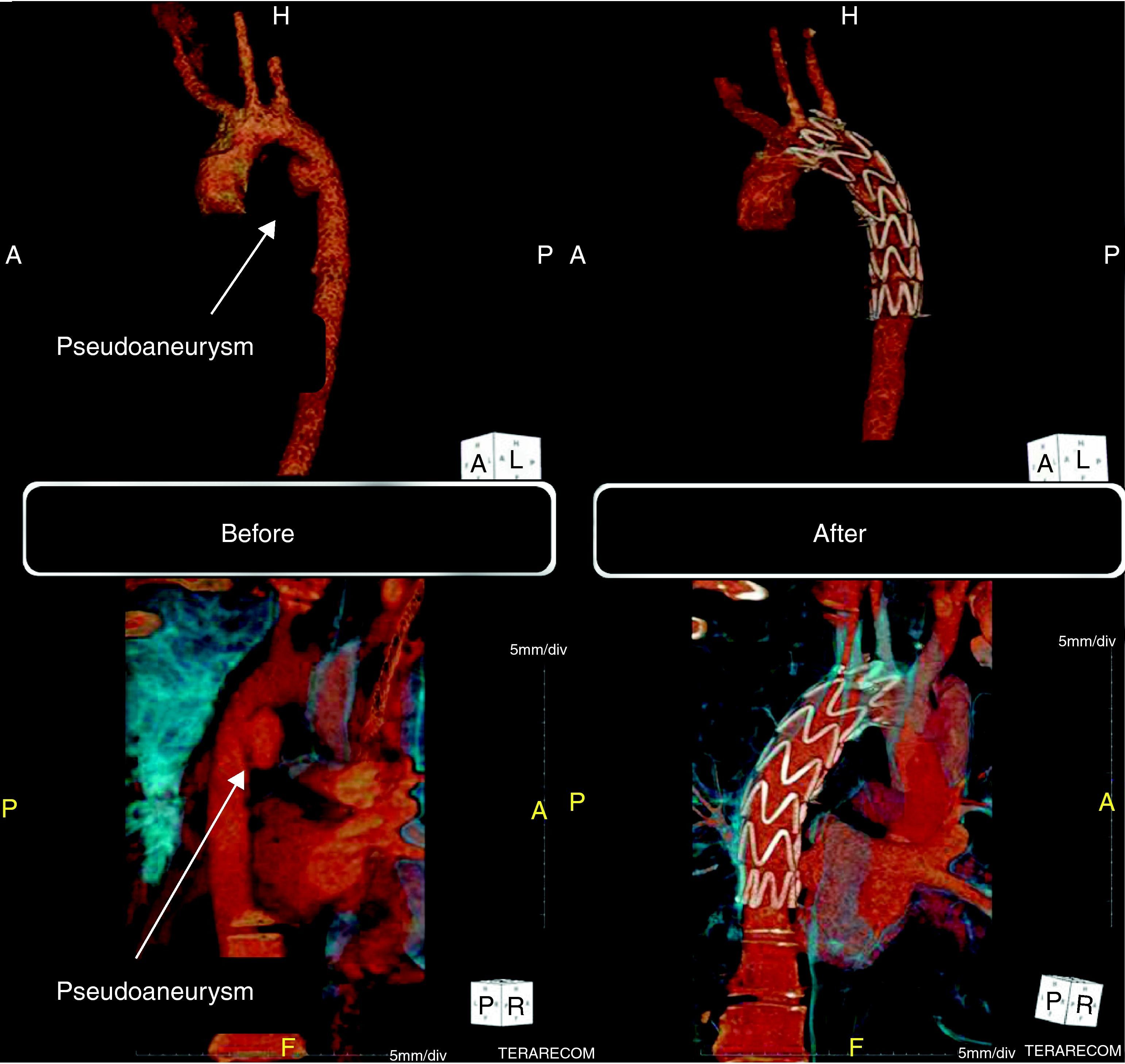
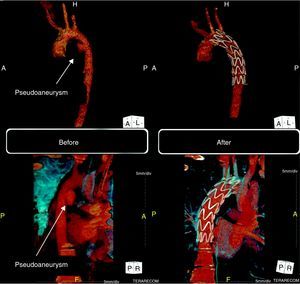
He underwent 64-slice thoracic CT angiography, which revealed a pseudoaneurysm of the descending thoracic aorta in the region of the aortic isthmus (Fig. 3, left). He also presented a small bilateral pleural effusion compatible with hemothorax.
Computed tomography (three-dimensional volume-rendering technique). Left: before the procedure, pseudoaneurysm of the descending thoracic aorta in the region of the aortic isthmus (15mm×18mm). Right: six months after the procedure, showing the endovascular stent-graft correctly positioned in the descending aorta and completely excluding the pseudoaneurysm.
Following discussion with the cardiothoracic surgery team, it was decided to adopt a percutaneous approach. Three days after the trauma a 100mm×22mm endovascular stent-graft (Valiant®, Medtronic) was implanted under general anesthesia and with right femoral access, which was closed with a Prostar® suture device (Abbott Vascular).
The clinical course was uneventful and the patient was discharged five days later.
Follow-up CT angiography six months after the procedure showed the endovascular stent-graft correctly positioned in the descending aorta, completely excluding the pseudoaneurysm (Fig. 3, right).
After almost four years of follow-up, the patient has no CV symptoms and imaging studies continue to show a good result.
DiscussionThe indications for TEVAR have widened in recent years to a growing number of thoracic aortic pathologies, particularly degenerative aneurysms (including those with associated rupture), acute type B aortic dissection, aortic trauma, intramural hematoma and penetrating aortic ulcers.1
TEVAR in thoracic aortic aneurysmThe most frequent indication for TEVAR is degenerative thoracic aortic aneurysm, as in the above case A. Several recent prospective studies have demonstrated that endovascular treatment is associated with lower morbidity and mortality than surgery,5,6 and may be preferable in such patients, particularly in acute symptomatic cases.7 These studies also showed it to be associated with lower perioperative mortality, reduced risk of paraplegia and paraparesis, and shorter hospital stay. The procedure has been shown to be safe as well as effective, although it is associated with increased risk of vascular access site complications.6
In the case presented, stent grafting excluded the aneurysm and significantly improved aortic remodeling on the follow-up CT angiogram six months after the intervention (Fig. 1).
The Society of Thoracic Surgeons’ Expert Consensus Document on endovascular treatment of descending thoracic aortic disease recommends that TEVAR be used in descending aortic aneurysms that are symptomatic and when the diameter of the descending aorta is over 65mm (or 60mm in centers with low mortality rates). The US guidelines for the diagnosis and management of thoracic aortic disease recommend that patients with aneurysms exceeding 55mm, even if asymptomatic, should be considered for endovascular treatment.2 The choice of TEVAR or surgery should be made on a case-by-case basis after discussion with a multidisciplinary team, considering not only the anatomical characteristics of the lesion but also the patient's age (the long-term durability of endovascular stent-grafts has not been fully established, but they last for at least 10 years) and comorbidities (with clear preference for endovascular treatment in older patients and those with more comorbidities), as well as the experience of the center.1,2
TEVAR in acute type B aortic dissectionThe conventional treatment for acute type B aortic dissection is medical, with invasive procedures (surgical or endovascular) reserved for cases complicated by progression of the dissection, imminent rupture, pain or hypertension refractory to therapy, or malperfusion syndromes. In complicated aortic dissection mortality can reach 50% with either medical or surgical treatment.8
Endovascular treatment of acute type B aortic dissection has emerged in recent years as a viable alternative to surgery that is less invasive, with 30-day mortality of less than 10%.9 The aim is to close the primary intimal tear between the true and the false lumen using a covered endovascular stent-graft. Case B above is an example of successful use of this technique, with significant clinical improvement and favorable aortic remodeling on imaging studies (increased diameter of the true lumen, reduction and thrombosis of the false lumen, reduced extent of the dissection and improved perfusion of the initially affected renal artery) (Fig. 2).
Endovascular treatment of acute type B aortic dissection is now a first-line option in complicated cases including progression of the dissection, refractory pain or organ malperfusion.1 However, its use in stable uncomplicated type B dissection is highly controversial. A recent randomized study showed that, while improving aortic remodeling, endovascular treatment did not significantly reduce mortality at two years compared to medical therapy alone.10
TEVAR in post-traumatic thoracic aortic injuryEndovascular stent-grafting is increasingly the treatment of choice for post-traumatic thoracic aortic injury,2,11 which is usually the result of deceleration injury, typically in traffic accidents, as in case C. These patients generally have multisystem involvement with various other injuries, and surgical treatment is associated with very high mortality.12 Several studies, including meta-analyses, have shown that TEVAR in such patients is associated with significantly less periprocedural mortality and complications than surgery.11,12 It can also be highly effective in traumatic aortic injury, as the case presented demonstrates. The 2010 US guidelines for thoracic aortic disease in fact recommend that TEVAR can be used in these patients.2
There are some special considerations concerning endovascular treatment of post-traumatic thoracic aortic injury. Firstly, these patients often have injuries to other organs, which frequently complicates a surgical approach. Such patients are usually younger and therefore have smaller caliber aortas, which may render TEVAR unfeasible since the smallest stent-graft currently available has a diameter of 23mm. There have been rare cases of stent collapse when the device is oversized.13 This once again highlights the importance of careful imaging studies before all procedures to ensure appropriate patient selection and planning of the intervention.
New clinical indications for TEVARType A aortic dissection has mortality of 1–2% per hour in the first 24–48 hours of evolution and around 50% at one week14 and hence emergent surgery is usually indicated.2 Nevertheless, perioperative mortality is high (10–35%), even in centers with considerable experience.
There have been several reports of successful endovascular treatment of retrograde type A aortic dissection15 and in highly selected patients with the entry tear in the ascending aorta.16,17 In our center we have successfully treated two cases of type A dissection with TEVAR. However, there are anatomical considerations with endovascular treatment, particularly the risk of occlusion of the supra-aortic vessels, and hence TEVAR in type A dissection is currently recommended only in highly selected patients18,19 who are unsuitable for surgery. Recent advances in this minimally invasive technique mean that it will probably be used more frequently in such patients.
In lesions involving the ascending aorta, another option is a hybrid technique combining conventional surgery with endovascular treatment. In such cases, the entry tear is closed by conventional surgery, and an endovascular stent-graft is placed in the descending aorta, when necessary, to correct conditions such as persistent peripheral malperfusion.19,20 This approach, which was used in three patients in our series, should be performed in conjunction with the cardiothoracic surgical team.
Endovascular treatment in cases of involvement of the aortic arch is much more complicated than descending aorta disease due to the risk of cerebral or upper limb ischemia. Stent grafting cannot exclude aneurysms or dissections that involve the origin of the brachiocephalic trunk or the left carotid artery unless measures have been taken to preserve circulation in these branches. One possibility is surgical debranching of these vessels before TEVAR, with or without bypasses between the supra-aortic trunks. These vascular procedures are relatively simple and do not necessarily entail exposure of the aneurysm, and hence allow alternative ways to revascularize the supra-aortic trunks.21 Branched stent-grafts have recently been developed to maintain the patency of the supra-aortic vessels.22
The risk of occluding the left subclavian artery is less important. When treating pathologies of the descending aorta, elective occlusion of this artery is often necessary to achieve firm fixation of the stent-graft. Although it was not required in any of the three cases presented here, it is generally well tolerated,23 without causing ischemia of the left arm. However, it is necessary to ensure that blood flow to the posterior region of the brain is not dependent solely on the left vertebral artery.
Possible complications associated with TEVARAlthough mortality and morbidity in TEVAR are low, the technique is associated with certain complications.
The most common complications in the immediate postprocedural period are related to the vascular access site. Endovascular stent-graft implantation requires a large arterial access site (usually femoral) since the smallest available delivery system is 22–24F (7.26–7.92mm) in diameter. However, the development of new techniques and percutaneous suture-mediated closure devices means that the procedure is now entirely percutaneous. Although no major vascular complications occurred in the cases reported here, surgical correction has on occasion been required, but this is increasingly rare as the operators gain experience, as seen in other studies.24 In the acute phase there is still a risk of paraplegia or paresis caused by occlusion of the intercostal arteries by the stent-graft resulting in spinal cord ischemia; this occurs in up to 7% of patients,25 although the risk is lower than with surgery.3
To minimize the risks, a multidisciplinary team is essential, including an interventional cardiologist, a cardiothoracic surgeon, a vascular surgeon (mainly to treat vascular access site complications), an anesthesiologist (for sedation and general anesthesia) and an intensive care cardiologist (to manage periprocedural complications and patient comorbidities).
The role of cardiac imaging in selection and follow-up of patients treated by TEVARCrucial to the success of endovascular intervention is expertise in cardiovascular imaging, particularly computed tomography. CT is required before the procedure for (i) accurate diagnosis of the type of aortic pathology; (ii) precise localization of the lesion and its relationship with the aortic branches, particularly the left subclavian artery and the celiac trunk; (iii) precise measurement of the diameters of the lesion and of the thoracic aortic lumen, especially where the stent-graft is to be fixed, in order to choose the correct size device; and (iv) assessment of the anatomy, size and tortuosity of the abdominal aorta and vascular access site.
After hospital discharge patients should undergo regular clinical and imaging evaluation. In our center the protocol includes low-dose CT angiography before discharge, at six months, one year and then annually. An alternative is magnetic resonance angiography, which has the advantage of not exposing patients to ionizing radiation.
Other complications may appear during follow-up, notably different types of endoleak26 (persistence of blood flow outside the stent-graft lumen), which occur in up to 10–20% of cases2 and necessitate reintervention.
ConclusionThese three cases add to the evidence that TEVAR is a safe and feasible therapeutic alternative in selected patients with different thoracic aortic pathologies, with relatively low mortality and morbidity and short hospital stay. However, there have been few randomized trials directly comparing different therapeutic options, and there is a particular lack of data on long-term outcomes.
Nevertheless, TEVAR is an extremely promising technique, and decisions on the best therapeutic approach to thoracic aortic pathologies should be taken on an individual basis by a multidisciplinary team, taking into consideration the patient's characteristics (particularly age, comorbidities and symptoms) and the results of imaging studies, especially the type, location, morphology and dimensions of the lesion.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fontes-Carvalho, R. Tratamento endovascular da patologia da aorta torácica: das indicações terapêuticas às possíveis complicações. Rev Port Cardiol 2012. doi:10.1016/j.repc.2012.01.008.