There is increasing interest in autoimmune diseases, especially their relationship with cardiovascular disease. Rheumatoid arthritis in particular has been considered an independent risk factor for coronary artery disease in recent years. Various studies have aimed to clarify important aspects of risk stratification and treatment options in patients with rheumatoid arthritis, and specific therapies are being studied that promise to reduce their long-term cardiovascular risk. We performed a wide-ranging review of the literature to highlight the importance of atherosclerotic and inflammatory mechanisms in coronary artery disease. We also suggest strategies for risk stratification and treatment of cardiovascular disease in patients with rheumatoid arthritis.
O interesse em doenças auto-imunes vem crescendo a cada ano, principalmente inter-relação com as doenças cardiovasculares. Especificamente a artrite reumatóide vem sendo considerada um fator de risco independente para doença arterial coronária nos últimos anos. Diversos estudos foram realizados recentemente com o objetivo de esclarecer pontos cruciais na estratificação de risco desses pacientes e no seu respectivo tratamento medicamentoso adequado. Novas terapias específicas da doença reumatóide ainda estão em estudo, e prometem reduzir o risco cardiovascular em longo prazo. Desse modo, realizamos uma revisão bibliográfica ampla, utilizando as principais bases de dados nacionais e internacionais, com o objetivo de salientar a importância de mecanismos ateroscleróticos e inflamatórios sobre a doença arterial coronária. Além disso, frente às atuais evidências, sugerimos estratégias de estratificação de risco e tratamento da doença arterial coronária em pacientes com artrite reumatóide.
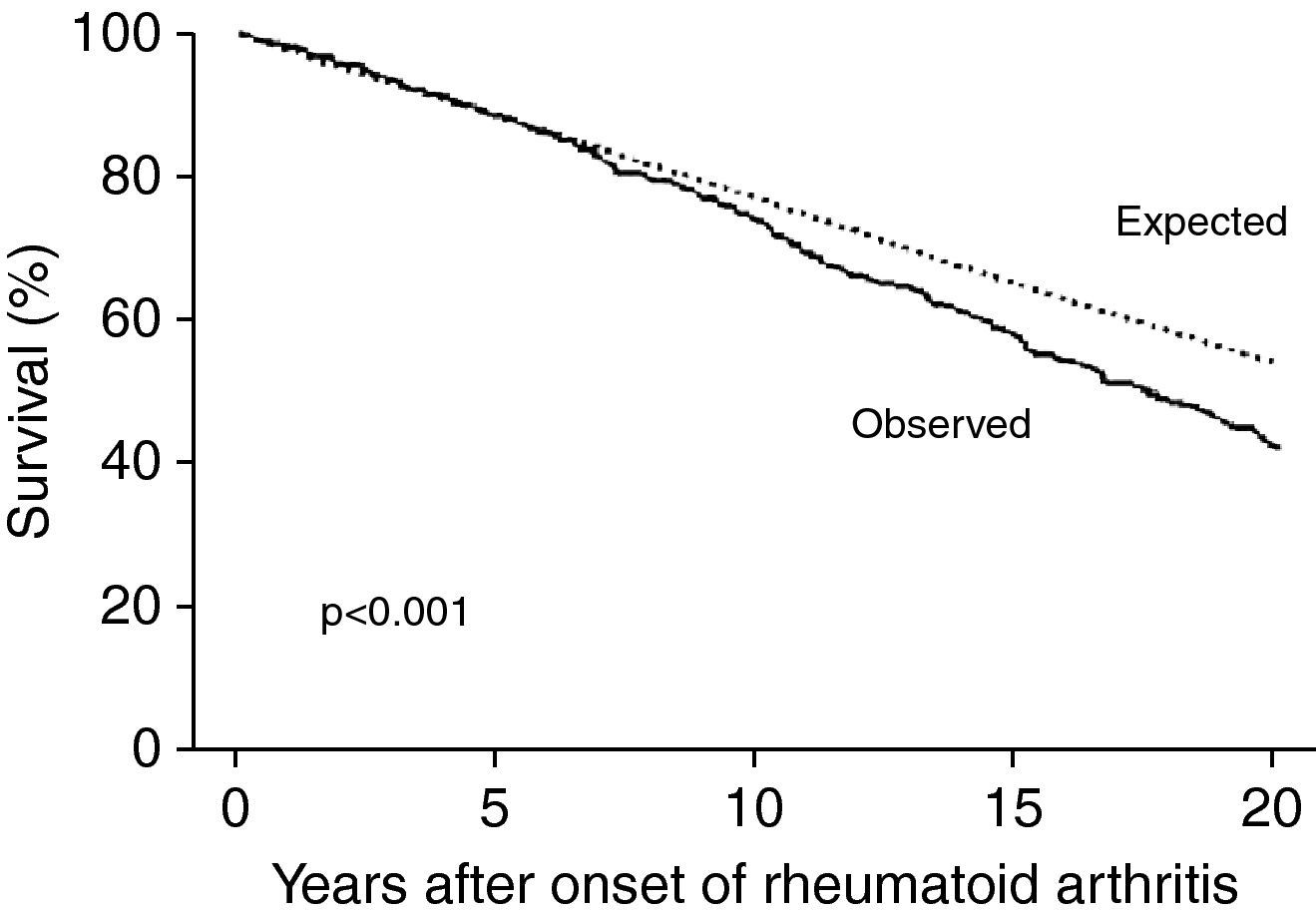
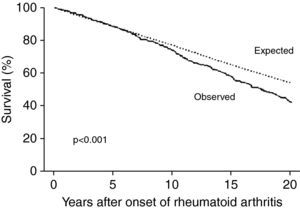
Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects 0.2–2.0% of the Brazilian population,1–3 mainly women, with peak incidence between the ages of 30 and 50 years.4 In recent years, studies have shown that the life expectancy of patients with RA is 5–10 years less than in the general population, due to their greater risk for cardiovascular disease (2–5 times higher than in the general population).2,3,5,6 Patients with RA thus have an equivalent absolute cardiovascular risk to those without RA who are 5–10 years older.6–8 Although the increased relative risk (RR) of cardiovascular events is more marked in younger patients with RA, older patients have more events in absolute terms, mainly due to premature development of coronary artery disease (CAD).2 A prospective cohort study in the US of 609 patients with RA with a mean follow-up of 14 years found greater risk of death in RA patients with RA than in those without RA of the same age and gender (Fig. 1) and a higher incidence of cardiovascular events (RR=1.6; p<0.001).8
Survival in individuals with rheumatoid arthritis compared to expected survival in the general population (adapted from Ref. 8).
In this review we summarize recent publications on the pathophysiology of atherosclerosis in patients with RA, examining the contribution of classical risk factors and inflammatory mechanisms that are specific to RA. We also analyze recent clinical studies on the occurrence of cardiovascular events in RA and discuss strategies for risk stratification and treatment of CAD in patients with RA.
Pathophysiology and atherosclerosis in RAThere is growing evidence from controlled clinical trials that patients with RA present more extensive atherosclerosis and coronary calcification than those without. This suggests that their higher incidence of cardiovascular events reflects greater and earlier atherosclerotic activity.9 Based on calcium scores, these patients have earlier and greater calcification of the aorta, carotid arteries and coronary arteries than controls.6 This increased calcification is more marked in males and younger age-groups, and more extensive calcification is associated with more severe RA.10
RA is considered an independent risk factor for intima-media thickening of the common carotid and femoral arteries, which correlates with the severity and chronicity of the disease.3,11 The reasons for this premature and more severe atherosclerosis have been the subject of considerable research. It is now accepted that the chronic inflammation found in RA plays an important part in the genesis and development of atherosclerotic plaques.10 Chronic inflammation, with elevated levels of circulating cytokines and autoantibodies, can destabilize atherosclerotic plaques and increase the risk of rupture. The substrate of CD4 and CD8 T lymphocytes found in unstable plaques is also seen in bone erosions in RA and in vasculitis. In addition, granzyme B, a product of macrophages involved in remodeling the extracellular matrix, is observed in large quantities in the synovia of patients with RA and in areas with ruptured atherosclerotic plaques, supporting the idea that systemic inflammation increases the risk of cardiovascular events.2
Other molecules known to be relevant to the pathophysiology of RA are also associated with atherosclerosis, including matrix metalloproteinases, proteolytic enzymes involved in joint destruction as well as in destabilization and rupture of vulnerable plaques. Similarly, elevated levels of coagulation factors and inflammatory cytokines such as fibrinogen, tissue factor, von Willebrand factor, plasminogen activator inhibitor, C-reactive protein (CRP), interferon-gamma, tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and interleukin-1 beta are found in both RA and CAD.5,11–13 Another molecule found at high concentrations in patients with RA is nitric oxide, which is produced by endothelial cells and is partly responsible for apoptosis and regulation of T lymphocytes in atherosclerotic plaques.12
Endothelial function is crucial to the development of atherosclerotic plaques.12 The endothelium plays an important role in regulating vascular tone, platelet activity and thrombogenesis. Endothelial dysfunction is one of the first stages in the development of atherosclerotic plaques, and can be triggered by systemic inflammatory states such as RA, mainly due to elevated levels of circulating cytokines. Some studies have indirectly shown endothelial dysfunction in RA, as individuals with RA present less endothelium-dependent vasodilation than those without.12
Importance of classical risk factors in ARBesides the atherogenic effects of chronic systemic inflammation, classical risk factors such as smoking, hypertension, diabetes and dyslipidemia also contribute to the genesis of atherosclerosis in patients with RA.14 Various studies have examined their prevalence and contribution to the genesis of cardiovascular disease in RA patients. No significant differences were found in the prevalence of hypertension, diabetes, dyslipidemia or obesity between individuals with and without RA in the initial stages of the disease; only smoking was more prevalent in patients with RA, which puts them at greater cardiovascular risk even before the appearance of inflammatory disease.6,11,12,15
Over the course of the disease, the prevalence of some risk factors changes, as patients with RA begin to present significantly less risk of developing hyperlipidemia and lower body mass index (BMI) than the general population.6,7 Little is known concerning the prevalence and type of dyslipidemia in these patients, different studies showing conflicting results. Some authors have found lower HDL cholesterol and higher LDL cholesterol in patients with RA11,16 compared to individuals without CAD or RA, but others have reported reduced total, LDL and HDL cholesterol and increased triglycerides, correlating directly with systemic inflammatory activity.2,11 In support of the latter finding, there is evidence that lipid profile has a paradoxical relationship with cardiovascular risk in patients with RA, with total and LDL cholesterol levels falling sharply in the three to five years preceding the onset of RA. Lower total and LDL cholesterol would thus indicate greater cardiovascular risk in these patients due to the presumed greater inflammatory activity associated with the disease. Furthermore, some studies have observed higher serum levels of lipoprotein(a), which may also be associated with greater risk, although this has not been proved.11,12
Unlike in the general population, a low BMI (<20kg/m2) in individuals with RA is linked to significant risk for cardiovascular death. As low BMI in patients with RA may indicate greater systemic inflammatory activity, this finding supports the importance of inflammation in these patients’ cardiovascular risk.6,7 Functional limitations due to bone lesions arising from the disease also tend to make individuals with RA more sedentary, which will also increase their cardiovascular risk.12
An important additional risk factor in patients with RA is increased insulin resistance and metabolic syndrome, which are directly related to carotid intima-media thickening and high coronary calcium score.2 Increased insulin resistance is related to dysfunction of pancreatic beta cells due to chronic inflammation.11 TNF-α is another important mediator in this process through its inhibition of glucose uptake in skeletal muscle.11
Curiously, although the prevalence of classical risk factors in patients with RA is similar to the general population, their impact is decidedly less: the risk of cardiovascular events related to such factors is considerably lower. This is probably because the cardiovascular risk arising from the presence of systemic inflammation in RA is so high that it dilutes the effect of other risk factors.2,6,7 The Framingham risk score, widely used in clinical practice, has been validated in patients with RA, and should be used in conjunction with noninvasive imaging studies, since a high Framingham risk score is a strong predictor of coronary calcification in patients with RA.9,16
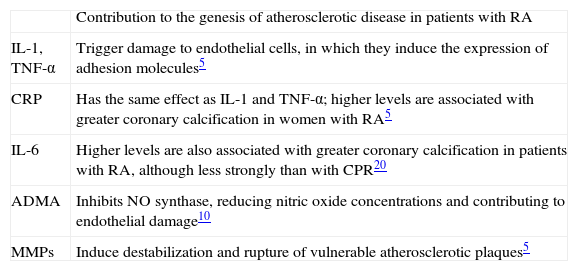
Inflammatory markers of cardiovascular riskAs pointed out above, chronic pro-inflammatory factors contribute to the development of atherosclerotic plaques in patients with RA (Table 1). In recent years attempts have been made to quantify such markers in order to predict cardiovascular risk in this population.6 Of these, erythrocyte sedimentation rate (ESR)>40mm/h, involvement of small and medium joints, bone erosions, rheumatoid nodules, vasculitis and rheumatoid pulmonary disease have been associated with greater risk of death.2,6–8,17,18
Inflammatory mediators associated with atherosclerosis in rheumatoid arthritis.
| Contribution to the genesis of atherosclerotic disease in patients with RA | |
| IL-1, TNF-α | Trigger damage to endothelial cells, in which they induce the expression of adhesion molecules5 |
| CRP | Has the same effect as IL-1 and TNF-α; higher levels are associated with greater coronary calcification in women with RA5 |
| IL-6 | Higher levels are also associated with greater coronary calcification in patients with RA, although less strongly than with CPR20 |
| ADMA | Inhibits NO synthase, reducing nitric oxide concentrations and contributing to endothelial damage10 |
| MMPs | Induce destabilization and rupture of vulnerable atherosclerotic plaques5 |
ADMA: asymmetric dimethylarginine; CRP: C-reactive protein; IL-1: interleukin-1; IL-6: interleukin-6; MMPs: metalloproteinases; RA: rheumatoid arthritis; TNF-α: tumor necrosis factor-α.
One extensively researched marker is rheumatoid factor (RF). In several studies high RF levels have been directly related to greater risk for death and combined endpoints including myocardial infarction (MI), heart failure (HF) and peripheral vascular disease.6,19 Gabriel7 showed that the RR of cardiovascular death in women with RA and negative RF was 1.59 (95% CI: 1.14–2.15) compared with 2.10 (95% CI: 1.73–2.51) in those with positive RF. In men, the results showed a similar trend, with RR of 1.59 (95% CI: 1.19–2.09) for positive RF and 0.81 (95% CI: 0.41–1.46) for negative RF. Some authors have suggested that even in the absence of RA, individuals with positive RF are at greater risk for cardiovascular events.19
Similar results have been found in patients with RA with antinuclear factor (ANF), which is also associated with greater risk for cardiovascular events.7,19
High levels of CRP, which is central to the pathophysiology of atherosclerosis, have also been shown to increase cardiovascular risk in patients with RA. In women, multivariate analysis has demonstrated that adjusting for serum CRP eliminates the relation between coronary calcification and RA, once again highlighting the importance of inflammatory activity as a risk marker in this disease.2,5,6,11
Besides ESR, RF, ANF and CPR, other markers associated with cardiovascular events include IL-6, soluble type 1 intercellular adhesion molecule, amyloid A, and fibrinogen.11,12 The importance of IL-6 as a mediator of cardiovascular events is indicated by the fact that adjusting for serum levels of this interleukin weakens the relation between coronary calcification and RA.20
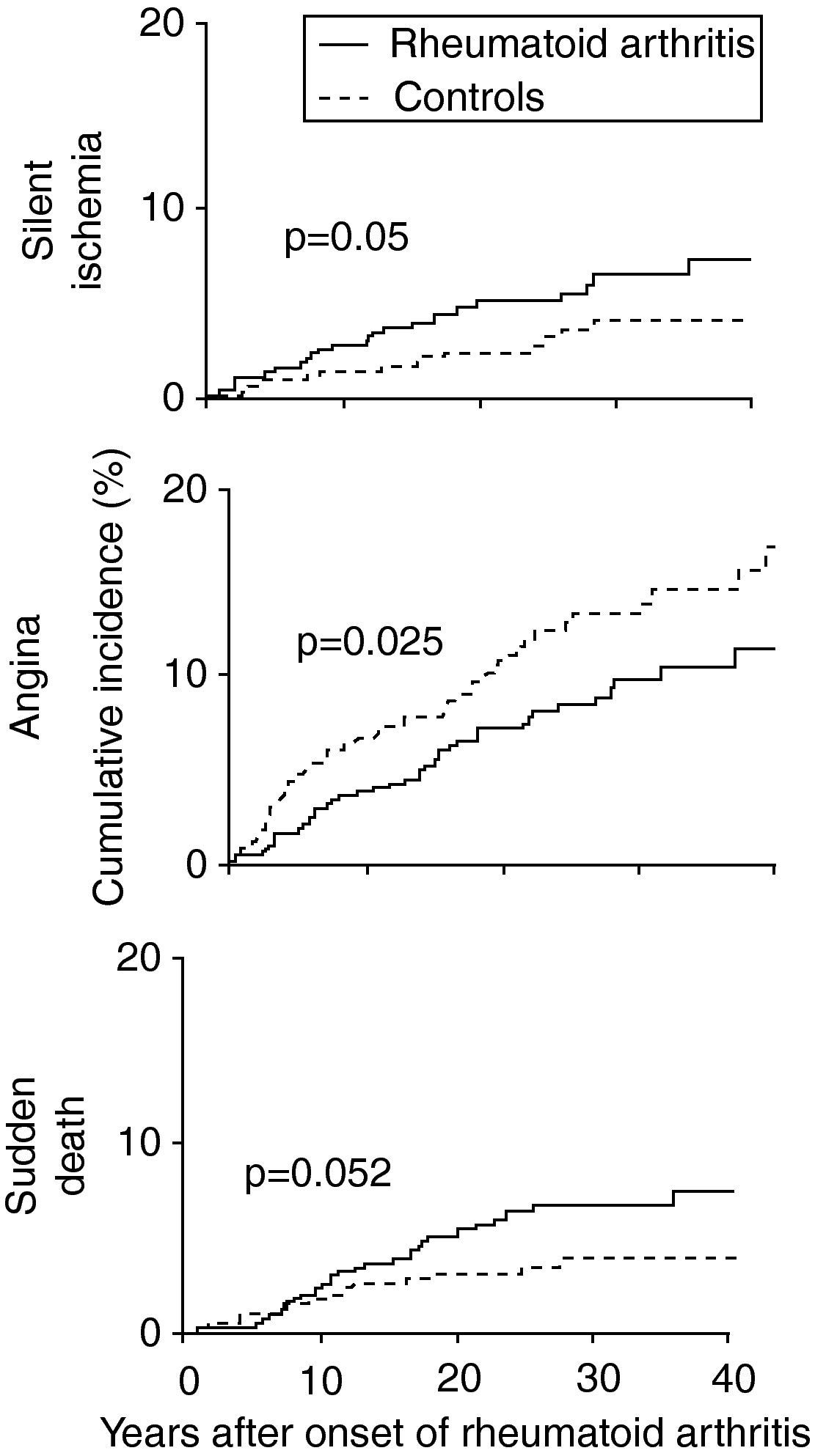
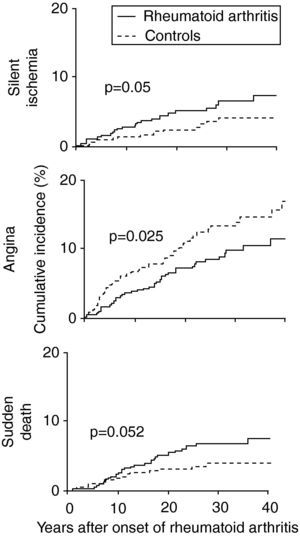
Characteristics of coronary artery disease in patients with RAPatients with RA have a high risk of acute coronary syndromes. A study based on the Rochester project,15 which followed 603 patients with RA, showed that in the two years before the appearance of RA these patients were more likely to suffer MI (odds ratio [OR] 3.17, 95% CI: 1.16–8.68) and silent ischemia (OR 5.86, 95% CI: 1.29–26.64) than gender- and age-matched controls.2,3,7,15,16,21 After diagnosis the higher risk for silent ischemia persisted (OR 2.13, 95% CI: 1.13–4.03), as well as for sudden death (OR 1.94, 95% CI: 1.05–3.55) (Fig. 2).3,7,15,21
Cumulative incidence of silent ischemia, angina and sudden death in patients with rheumatoid arthritis compared to controls (adapted from Ref. 15).
The longer the duration of the disease, the greater the risk of coronary events, particularly more than 10 years after diagnosis.16 This may be explained by increased production of pro- and anti-inflammatory cytokines in individuals with RA, which alters their perception of pain.7,15 It may also be linked to their reduced mobility due to joint disease, which prevents them from taking strenuous exercise that would trigger angina, or failure by the physician or the patient to give due weight to anginal pain, attributing it to the musculoskeletal effects of RA.7,11
Unlike all in vivo anatomical studies, an autopsy study found no differences in extent and degree of coronary stenosis between those with and without RA. Unexpectedly, fewer individuals with RA had three-vessel disease than those without (32% vs. 61%; p<0.018), and they tended to have less severe atherosclerotic disease.22 However, greater inflammatory activity was observed in atherosclerotic plaques in the circumflex and anterior descending coronary arteries, 48% of those in the anterior descending artery being classified as vulnerable, compared to only 22% in those without RA (p=0.018).7,22 Once again, this study suggests that the etiology of CAD in patients with RA is mainly related to inflammation.7,22 It should be stressed that this autopsy study is the only one aimed specifically at analyzing atherosclerosis in RA, and its sample size was small, which limits generalization of its results.
Extracoronary disease in patients with RAVarious studies have demonstrated carotid intima-media thickening in patients with RA, and atherosclerotic plaque progression tends to be greater (and directly related to serum CRP and ESR). Carotid plaques are up to three times more likely in individuals with RA than in those without RA, even when adjusted for age, gender and other cardiovascular risk factors.16,23
Unlike what is observed with CAD, no large cohort study on RA has shown a greater incidence of stroke or peripheral arterial disease.7,16
The relationship between RA and heart failureAs with CAD, patients with RA are at higher risk of developing HF than the general population.7,18,24 The cumulative incidence of HF over 30 years of follow-up is up to 34% in patients with RA compared to 25% in those without RA (p<0.001), even after adjustment for other cardiovascular risk factors.7,13,15,18,24 However, the risk of HF appears to be directly related to the presence of positive RF (OR 2.59, 95% CI: 1.95–3.43) compared to those with negative RF (OR 1.28, 95% CI: 0.93–1.78).7,15,18
A prospective cohort study with a mean follow-up of 15 years by Maradit-Kremers et al.17 in 575 patients with RA showed that in the six months before the appearance of HF, 23% of patients presented ESR>40mm/h, considerably more than the 10% among the other subjects. According to the authors, this suggests that, as with CAD, inflammation may be involved in triggering HF in patients with RA.17
It has also been observed that the prognosis of HF in patients with RA is worse than in those without RA. In the 30 days following diagnosis of HF, mortality in patients with RA was 16%, compared to 7% in controls (p<0.001), with a similar trend at 6-month follow-up (OR 1.94, 95% CI: 1.17–3.23).7
Finally, patients with RA are more likely to develop HF with preserved left ventricular ejection fraction (OR 2.57, 95% CI: 1.20–5.49).7,25 A recent study showed that after a mean follow-up of 25 years, 31% of patients with RA had been diagnosed with HF with preserved (>50%) ejection fraction, compared to 26% of age- and gender-matched controls (OR 1.6, 95% CI: 1.2–2.4). Moreover, individuals with RA had significantly lower left ventricular mass index, higher pulmonary artery pressure and greater left atrial volume. The duration of RA and serum IL-6 levels were independently and directly related to greater prevalence of diastolic HF in patients with RA.25 It should be noted that pulmonary hypertension secondary to RA is difficult to characterize in these cases, but is extremely rare.25
Diagnostic techniques for risk stratification in RAImaging techniques to stratify risk for CAD in patients with RA have been widely studied,9,14,20,23,26 particularly coronary computed tomography (CT) angiography. Patients with RA generally present greater coronary artery calcification, and hence higher calcium score, this being directly related to disease duration and ESR.14,16 In a study by Wang et al.23 of 85 patients with RA who underwent coronary CT angiography, these patients presented higher RR of calcification of the aorta (OR 19.5, 95% CI: 8.0–47.6), carotid arteries (OR 5.7, 95% CI: 1.7–18.7) and coronary arteries (OR 5.0, 95% CI: 2.2–11.1) than age- and gender-matched individuals without RA. Of those with RA aged over 60, 90% had diffuse arterial calcification, mainly of the thoracic aorta.23
Other studies have shown that coronary calcification detected by coronary CT angiography in RA patients is more prevalent in men than in women (p=0.012), while their mean calcium score is 53 units higher than in those without RA who also have an elevated score (p=0.017).20
Intima-media thickness measured by carotid ultrasound is another early marker of atherosclerosis, correlating closely with the presence of CAD in patients with RA.10,16 As with CT angiography, echocardiography reveals greater intima-media thickness in patients with RA, the more so the longer the disease duration.10
The effects of drug therapy or RA on cardiovascular riskIt is difficult to analyze the effects on cardiovascular risk of different drug classes used in the treatment of RA because of confounding factors such as indications and contraindications for particular treatments. The contribution of most drugs is therefore still the subject of debate.7
Prolonged corticosteroid therapy is a potential cause of premature atherosclerosis, insulin resistance, hypertension and raised total and LDL cholesterol and triglycerides, all of which increase cardiovascular risk in patients with RA.2,7,11 However, some studies have shown that in patients taking high cumulative doses of corticosteroids (>7000mg), it was the presence of positive RF that was associated with greater risk for events, and corticosteroid use in itself was not an independent risk factor.7,27 Furthermore, in patients with RA and a history of CAD, corticosteroid therapy appears to reduce cardiovascular risk, implying that control of inflammation may be the most important factor.2,7,11 Corticosteroids appear to lower homocysteine levels, which may be another way in which they decrease cardiovascular risk.11 However, another study demonstrated increased carotid plaque formation and peripheral arterial disease in RA patients taking over 16000mg of corticosteroids, highlighting the need for caution when prescribing high dosages.16
Methotrexate is another drug that appears to affect cardiovascular risk in RA, although its precise effect is still unknown.7,28–30 In the most frequently cited study in the literature, its use was associated with reduced risk, mainly shown by the considerably lower incidence of MI in those taking it (OR 0.3, 95% CI: 0.2–0.7).7,30–32 Other studies have shown a reduction in peripheral arterial disease in RA patients taking the drug,7,11,16 and levels of HDL cholesterol and apolipoprotein (a) improve after 12 months of methotrexate therapy.28 However, in another study of patients with RA and known CAD, the use of methotrexate was associated with higher mortality (OR 3.4, p=0.0054), highlighting the need for further studies in this area. Although the precise mechanism is unclear, methotrexate can lead to hyperhomocysteinemia, which would increase cardiovascular risk, and is directly toxic to endothelial cells, induces oxidation of LDL cholesterol, and has prothrombotic effects.11
Recent evidence indicates that TNF-α blockade improves control of systemic inflammation and has direct effects on the endothelium,2,10,12,13,33 although there are inconsistencies. Some studies suggest that endothelial function improves as systemic inflammation is controlled,2,10,33 while others report increased coronary flow reserve after 24 months of treatment.10 Although one study showed long-term worsening of lipid profile, with reduced HDL and increased total cholesterol, another study of 80 patients showed no significant changes in lipid profile at the end of 48-month follow-up.2,34
There are uncertainties regarding the use of statins in RA. Their pleiotropic effects potentially protect the endothelium, and it is widely accepted that they are indicated for patients with RA and elevated LDL cholesterol. However, it is not known whether the benefits of these drugs in the general population due to reductions in LDL cholesterol can also be expected in chronic inflammatory states.2 Ezetimibe and simvastatin have been tested in patients with RA, and have resulted in reduced systemic inflammation and concomitant improvement in endothelial function.10,35
Hydroxychloroquine reduces the long-term risk of developing diabetes in RA patients.2 Sulfasalazine, leflunomide and chloroquine have been tested in a large study on patients with RA, and a significant reduction in cases of MI was seen in those receiving the drugs.16,32
What cardiologists should know about RAAs pointed out above, cardiovascular risk in patients with RA differs from the general population, and there are no well-established guidelines for their treatment. It is thus not uncommon for a cardiologist assessing a patient with RA to be unsure of certain aspects. Current evidence suggests that the following recommendations may be helpful:
- (1)
RA should be considered an independent cardiovascular risk factor, and the physician should treat such patients as being 5–10 years older than they in fact are7;
- (2)
Classical risk factors for atherosclerosis such as smoking, dyslipidemia, hypertension, diabetes and obesity should be treated and controlled in the same way as with any other patient, although these risk factors are less important in patients with RA, probably because of other factors that modify their risk11;
- (3)
The Framingham risk score has been validated for risk stratification of patients with RA and should be used, since it has high positive predictive value for coronary calcification in RA patients classified as high-risk9;
- (4)
Noninvasive imaging techniques including coronary CT angiography and carotid ultrasound have been validated in this population and should be used to aid risk stratification. The indications are the same as for the general population,36 except that since subclinical atherosclerosis is more prevalent in patients with RA, they should be used earlier;
- (5)
There are no specific indications for the use of statins or aspirin for primary prevention in patients with RA, and the same recommendations should be adopted as for other patients36;
- (6)
The cardiologist should routinely request tests and be alert for symptoms or signs that indicate systemic inflammation, such as arthritis or elevated CRP and ESR. Consultation with the rheumatologist is essential in all cases and patients with signs of inflammatory activity should be rapidly referred6,7,19;
- (7)
Patients should be tested for factors known to be markers of high risk for coronary events, such as RF and ANF, and if positive should be closely monitored, with noninvasive diagnostic tests to improve risk stratification6,7,19;
- (8)
Corticosteroids should be prescribed when indicated to control systemic inflammatory activity, but doses should be kept as low as possible, particularly in the long term7,27;
- (9)
Drugs such as chloroquine, leflunomide, methotrexate and TNF-α antagonists can be used without restrictions, since current evidence on clinical outcomes indicates that complete control of inflammatory activity is more important than possible side effects7;
- (10)
Patients with RA should be regularly reassessed for symptoms of HF and given appropriate treatment when necessary. It should be borne in mind that most cases are of diastolic HF only.25
Rheumatoid arthritis should be considered an independent risk factor for coronary artery disease. There have been considerable advances in recent years in our knowledge of autoimmune diseases and cardiovascular risk. However, much of the evidence concerning cardiovascular disease in patients with RA comes from observational studies, and further research is needed. Particular strategies for risk stratification and treatment are required for these patients and new guidelines should be issued based on current evidence. In general, appropriate control of systemic inflammation is the principal factor in reducing risk for coronary events and should always be the goal.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: de Matos Soeiro A, et al. Artrite reumatóide e doença cardiovascular: o que sabemos e o que podemos fazer pelo paciente na atualidade? Rev Port Cardiol. 2012. doi:10.1016/j.repc.2012.01.005.