Heart failure (HF) still portends a grim prognosis.1 For many, pump failure may progress, leading to cardiogenic shock (CS), a dismal event.2,3 In these cases, mechanical circulatory support (MCS) can restore end-organ perfusion and serve as a bridge to recovery.2,3 Nonetheless, myocardial recovery may be insufficient, requiring a different, long-term strategy. Heart transplantation (HT) remains the gold standard for advanced HF, but the limited donor pool may preclude transplantation.4 Long-term left ventricular (LV) assistance devices, such as the HeartMate 3™ (HM3), have emerged as a complementary strategy to HT.
We report the challenging case of a 43-year-old male, with previous above-the-knee amputation and anthracycline exposure due to left femur osteosarcoma, presenting with new-onset fatigue. Blood pressure was normal (140/90 mmHg) despite marked sinus tachycardia (130 bpm). Workup revealed an N-terminal pro-brain natriuretic peptide (NT-proBNP) level of 4444 pg/ml and high-sensitivity cardiac troponin I 18 ng/l. The echocardiogram showed a dilated, diffusely hypokinetic left ventricle (ejection fraction 15%); severely compromised cardiac output (LV outflow tract pulsed-wave Doppler velocity time integral 3 cm); and right ventricular (RV) dysfunction (tricuspid annular plane systolic excursion 10 mm). He rapidly progressed to severe hypotension with stage C cardiac shock on the Society for Cardiovascular Angiography and Interventions (SCAI) classification (peak lactate 4.3 mmol/l), and was accordingly admitted to the intensive care unit (ICU). Levosimendan and norepinephrine were initiated and lactate fell to 2.1 mmol/l. Despite absence of end-organ dysfunction and achieving stability, his worrying echocardiographic findings led to the decision to preemptively proceed to awake venoarterial (VA) femorofemoral extracorporeal membrane oxygenation (ECMO) within 24 h of admission as a bridge to decision. Angiography excluded coronary disease. While RV improvement was observed (non-distended right ventricle with acceptable systolic function after clamping of the venous cannula), there was no significant LV recovery, precluding weaning from MCS. However, ECMO-related complications (oozing at the cannula site) occurred. Thus, after heart team discussion, the patient was listed for HT with consideration of an HM3 if no donor was available within a reasonable time. After seven days of listing, the decision was made to implant an HM3. Somewhat surprisingly, severe RV failure was observed post-ECMO decannulation and starting the HM3 pump, requiring placement of an RV assist device (RVAD). Progressive RV improvement was later observed, permitting explantation of the RVAD after 13 days. The patient was finally discharged home after two months, having regained optimal functional status. Myocardial biopsy (obtained during HM3 implantation) showed no signs of active myocarditis.
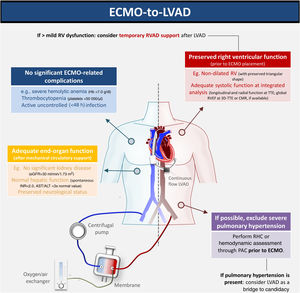
We report the first-ever Iberian case of bridge-to-bridge (VA-ECMO to HM3) as bridge to transplant. The decision regarding which bridging strategy is preferable when the patient is unable to undergo MCS weaning is controversial. Given the long waiting-list times for HT, the HM3 may be a suitable alternative. However, this strategy merits caution. The expected RV improvement during RV unloading in ECMO and simple bedside tests (such as transient circuit clamping) may be misleading in excluding the need for an RVAD. During surgery, optimizing RV preload and inotropic support are key steps in preventing RV failure. Intraoperative transesophageal echocardiography and serial echocardiography post-surgery must also be systematically performed. An integrated, multidisciplinary discussion is essential to determine the optimal management strategy (Figure 1).
Optimal patient assessment for ECMO-HM3 bridge to transplant. 3D: three-dimensional; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CMR: cardiac magnetic resonance; ECMO: extracorporeal membrane oxygenation; eGFR: estimated glomerular filtration rate; Hb: hemoglobin; HM3: HeartMate 3™; LVAD: left ventricular assist device; PAC: pulmonary artery catheter; RHC: right heart catheterization; RV: right ventricular; RVAD: right ventricular assist device; RVEF: right ventricular ejection fraction; TTE: transthoracic echocardiography.
In our case, several factors led us to opt for advanced HF therapies and HM3 after ECMO bridge. Our patient was young, had no significant comorbidities (his previous osteosarcoma was cured) and no suspicion of pulmonary hypertension. No reversible cause of CS was found, and so a diagnosis of chemotherapy-induced cardiomyopathy was assumed, leading to a low probability of myocardial recovery. Also, unlike Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) level 1–2, there was no irreversible end-organ dysfunction, since the patient's CS was quickly recognized and promptly managed.
Managing CS is challenging. Our case illustrates that MCS may be appropriate in selected patients in SCAI stage C/INTERMACS levels 2–3 and should not be reserved for those with deteriorating status (e.g. SCAI stages D or E; INTERMACS level 1). Our patient fell in the former group, which may explain his favorable outcome. Timely referral to an experienced center with a dedicated cardiac ICU, HF/HT specialists and cardiac surgeons is critical to provide the best chance of survival.5 Bridge-to-bridge (VA-ECMO to HM3) may be a reasonable option in cases such as that presented herein.
Authorship contributionSM wrote the first draft of this article; BR and CA incorporated feedback in subsequent drafts and revisions; MSP, GC, MR, CB, CS, AT, AV, TN, JPN and MM contributed to revisions and reviewed the final draft; SM composed the figure and submitted the final version of this article, on behalf of all the authors.
Conflicts of interestThe authors have no conflicts of interest to declare.