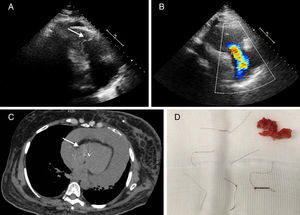
Advances in magnetic resonance imaging (MRI) over the past two decades have led to it becoming an increasingly attractive imaging modality. With the growing number of patients treated with permanent implanted or temporary cardiovascular devices, it is becoming ever more important to clarify safety issues with regard to MRI examinations in patients with these devices. A 57-year-old woman was admitted to the emergency department with a one-month history of progressive anasarca. Her medical history included subarachnoid hemorrhage and a deep vein thrombosis in the same hospitalization period, treated with inferior vena cava (IVC) filter placement (ELLA®, Hradec Králové, Czech Republic). The initial echocardiographic assessment showed hyperechoic linear images in the right chambers (Figure 1A) and severe tricuspid regurgitation (Figure 1B). Chest computed tomography confirmed the hypothesis of IVC filter migration, demonstrating intracardiac metallic fragments (Figure 1C). After clinical stabilization with intravenous diuretics, the patient underwent cardiac surgery and thrombus and IVC filter fragments were removed (Figure 1D), in addition to tricuspid valve repair, followed by successful recovery. After a thorough review of the patient's clinical history, it was discovered that she had recently undergone a brain MRI in another institution (about two months before symptom onset), without mentioning implantation of the IVC filter about nine months before. Although this MRI had been performed late, it was probably the cause of IVC filter migration, highlighting the safety concerns with this examination in patients with this device.
The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.