Atrial fibrillation is the most common arrhythmia in adults and its prevalence is growing rapidly. It has been shown that AF is associated with increased risk of heart failure, ischemic and hemorrhagic stroke, and mortality. Hence, there is growing interest among researchers in seeking preventive and therapeutic interventions regarding AF. In recent decades, it has been suggested that statins may decrease the incidence of AF and may also decrease its recurrence after cardioversion and catheter ablation. These effects are thought to be mediated by different mechanisms such as modulating inflammation, altering the properties of transmembrane ion channels, interfering with activation of matrix metalloproteinases, and acting on endothelial function. In this article, we review and update current knowledge about the role of statins in primary and secondary prevention of AF in general and specific populations.
A fibrilação auricular (FA) é a arritmia mais comum em adultos e a sua prevalência está a crescer rapidamente. Foi demonstrado que a FA está associada a um risco acrescido de insuficiência cardíaca, acidente vascular cerebral isquémico e hemorrágico e mortalidade. Assim, há um interesse crescente entre os investigadores na procura de intervenções preventivas e terapêuticas em relação à FA. Nas últimas décadas, tem sido sugerido que as estatinas podem diminuir a incidência de FA e também diminuir a sua recorrência após a cardioversão e ablação do cateter. Pensa-se que estes efeitos são mediados por diferentes mecanismos, tais como a modulação da inflamação, a alteração das propriedades dos canais de iões transmembrana, a interferência com a ativação das metaloproteinases matriciais e a atuação sobre a função endotelial. Neste artigo, revemos e atualizamos os conhecimentos atuais sobre o papel das estatinas na prevenção primária e secundária da FA em populações gerais e específicas.
Atrial fibrillation (AF) is the most common arrhythmia in adults and its prevalence is growing rapidly. It is estimated that the number of affected individuals will increase three-fold by 2050.1 Many studies have shown that AF is associated with increased risk of heart failure, ischemic and hemorrhagic stroke, and mortality.2 Hence, there is growing interest among researchers in seeking preventive and therapeutic interventions regarding AF.
Statins are a group of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors with pleiotropic properties independent of their lipid-lowering effects.3 Statins have been shown to exert multiple beneficial effects in the cardiovascular system, including plaque stabilization and reductions in myocardial infarction and mortality.4,5 In recent years, it has been suggested that statins may decrease the incidence of AF and may also decrease its recurrence after cardioversion and catheter ablation.6 These effects are thought to be caused by numerous pathways through which statins act on atrial structural and electrical remodeling, such as modulating inflammation, altering the properties of transmembrane ion channels, interfering with activation of matrix metalloproteinases, and acting on endothelial function.7,8 Due to the importance of the issue and conflicting results, many studies are published annually regarding the role of statins in the prevention and treatment of AF. In this article, we review and update current knowledge about the role of statins in primary and secondary prevention of AF.
MethodsWe searched Medline, Scopus, and ISI Web of Knowledge for all studies published from January 1980 through December 2019 regarding statins and AF. We conducted text searches with the terms ‘statin’ and ‘atrial fibrillation’. We also manually searched references from selected original research, clinical trials, meta-analyses, and recent review articles. Data were extracted from articles that were published in English.
Primary prevention of atrial fibrillationStatins are currently the cornerstone of medical treatment in patients with atherosclerotic cardiovascular disease. In addition, they are used in multiple other settings depending on the patient's lifetime risk. This has resulted in numerous longitudinal studies reporting the effects of statin therapy on the incidence of AF in different patient subgroups.
Registry-based studies in Taiwanese and Danish populations assessed the role of statins in the prevention of new-onset AF in the general population.9,10 A study by Hung et al., which followed 171 885 Taiwanese patients older than 50 years for nine years, showed that statin therapy reduced the risk of AF by 28% (adjusted hazard ratio [HR]: 0.72; 95% confidence interval [CI]: 0.68-0.77).9 Additionally, subgroup analysis showed that individuals with CHA2DS2-VASc or CHADS2 scores of 1 or more derive greater benefit from statin use (p<0.001). The Danish study (565 044 subjects) showed similar beneficial effects and emphasized the duration of statin use, with a lower incidence of AF reported in patients on long-term statin therapy.10
A Danish registry of 89 703 patients with first-time acute myocardial infarction (mean follow-up of 5.0±3.5 years) registered new-onset AF in 5698 (10%) vs. 5010 patients (15%) among those receiving and not receiving statin treatment, respectively. After adjustment for multiple variables, the results showed a significant reduction in new-onset AF (HR: 0.84, 95% CI: 0.80-0.87, p<0.001) in statin users.11 Additionally, in a cohort study of the HERS trial on 2673 postmenopausal women with known coronary artery disease (CAD), statin therapy was associated with a 55% risk reduction in the incidence of AF over a mean of 4.1 years (HR: 0.45, 95% CI: 0.25-0.74, p=0.002).12 Furthermore, Zhou et al. conducted a meta-analysis on 10 cohort studies including 193 839 patients with CAD13 and observed a significant reduction in the occurrence of AF with statin use (odds ratio [OR]: 0.65; 95% CI: 0.57-0.74, p<0.0001). The benefit was even more marked in patients with acute coronary syndrome (OR: 0.62; 95% CI: 0.51-0.75, p<0.0001).
Congestive heart failure (CHF) is a major risk factor for the development of AF, with studies showing a 5 to 10-fold higher risk of AF in this population.14 The beneficial effect of statins in primary prevention of AF in this subgroup has been demonstrated in several studies. In a prospective study of 25 268 individuals with left ventricular ejection fraction <40%, use of statins was associated with a 31% reduction in the occurrence of AF.15 Similarly, the GISSI-HF trial, although it demonstrated no survival benefit in patients with CHF, showed an 18% reduction in the risk of AF among patients with CHF receiving rosuvastatin.16 However, this significant effect was observed only after adjustment for clinical variables, laboratory examinations, and background medical therapy. The SCD-HeFT trial investigators also reported that statin use reduced the risk of AF by 28%, which, interestingly, was comparable with that of amiodarone.17
Atrial fibrillation/flutter has been reported to occur in 7-27% of chronic kidney disease (CKD) patients.18 In a Taiwanese registry of 70 445 patients including 6767 patients with CKD (mean follow-up of 4.0 years),19 a 57% reduction in risk of AF was observed in patients with CKD on continued statin use (adjusted HR: 0.43; 95% CI: 0.27-0.68). Another registry-based study enrolled 113 191 patients on hemodialysis and observed that after a median follow-up of 4.29 years the incidence of AF was lower in the statin group than in controls (2.4% vs. 4.9%, p<0.001).20
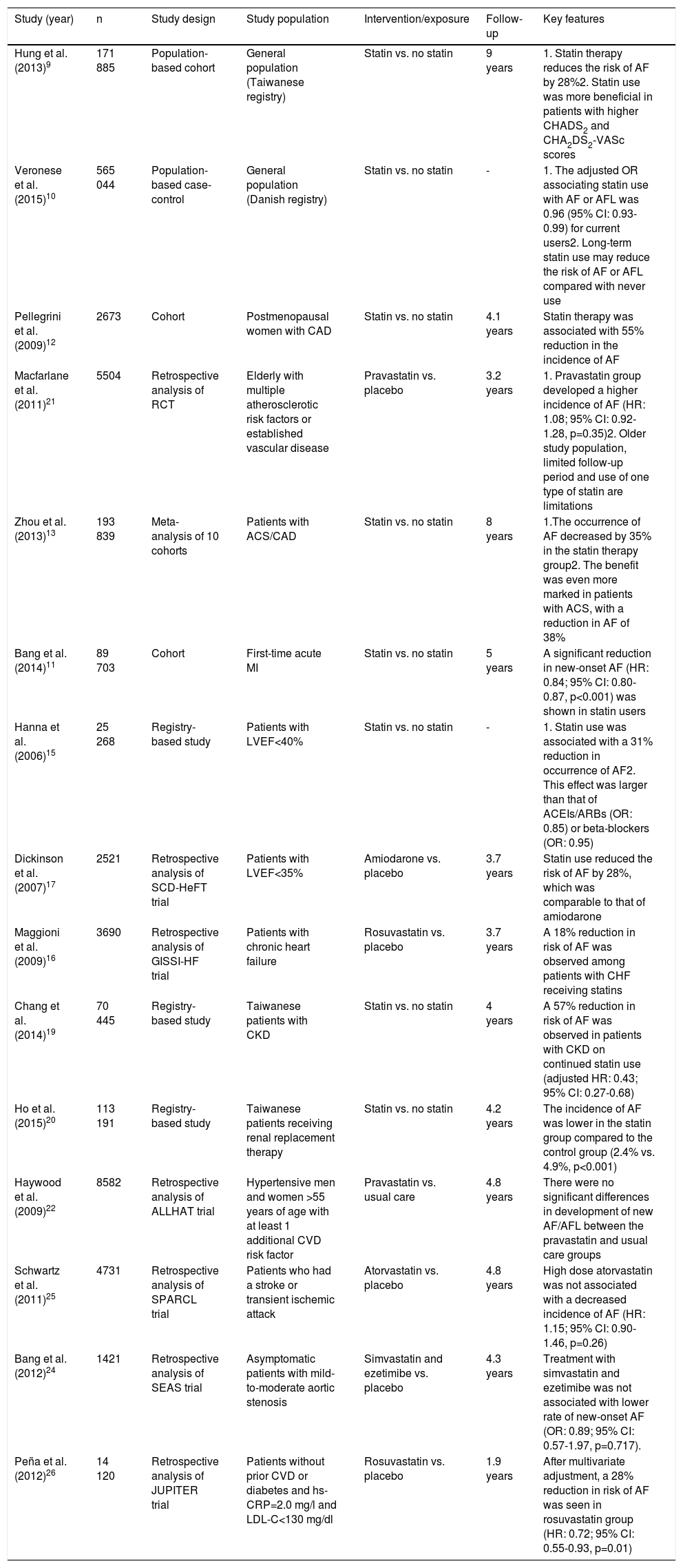
In addition to the above-mentioned longitudinal studies on the role of statin therapy in reducing the risk of new-onset AF, there have been multiple randomized controlled trials (RCTs) assessing the efficacy of statin therapy in primary prevention of AF as a secondary endpoint of the study. A retrospective analysis of the PROSPER trial, which enrolled 5504 individuals between 70 and 82 years of age with a history of multiple atherosclerotic risk factors or established vascular disease, showed no significant effect of pravastatin on primary prevention of AF.21 This non-significant effect of statin therapy on primary prevention of AF was further supported in trials enrolling patients with different risk factors for CAD, including the ALLHAT and WOSCOPS trials, which enrolled men with moderate hyperlipidemia and high-risk hypertensive patients, respectively.22,23 Similarly, other randomized trials enrolling patients with mild to moderate aortic stenosis (SEAS24) or patients with a prior history of stroke or transient ischemic attack (SPARCL25) showed no significant reduction in the incidence of new-onset AF in the statin group. The only RCT that showed any effect was the JUPITER trial, in which there was a 28% reduction in the risk of AF with the use of rosuvastatin in individuals without prior cardiovascular disease.26 In a meta-analysis by Fauchier et al. of nine RCTs, statin therapy had no beneficial effect on primary prevention of AF (pooled OR: 1.00, 95% CI: 0.86-1.15).27 Hence, despite consistent findings from longitudinal studies suggesting the beneficial effect of statins in reducing the risk of new-onset AF, analysis of data from RCTs assessing the efficacy of statin therapy for primary prevention of AF has shown that statins have no such effect (Table 1).
Studies assessing the efficacy of statins in primary prevention of atrial fibrillation.
| Study (year) | n | Study design | Study population | Intervention/exposure | Follow-up | Key features |
|---|---|---|---|---|---|---|
| Hung et al. (2013)9 | 171 885 | Population-based cohort | General population (Taiwanese registry) | Statin vs. no statin | 9 years | 1. Statin therapy reduces the risk of AF by 28%2. Statin use was more beneficial in patients with higher CHADS2 and CHA2DS2-VASc scores |
| Veronese et al. (2015)10 | 565 044 | Population-based case-control | General population (Danish registry) | Statin vs. no statin | - | 1. The adjusted OR associating statin use with AF or AFL was 0.96 (95% CI: 0.93-0.99) for current users2. Long-term statin use may reduce the risk of AF or AFL compared with never use |
| Pellegrini et al. (2009)12 | 2673 | Cohort | Postmenopausal women with CAD | Statin vs. no statin | 4.1 years | Statin therapy was associated with 55% reduction in the incidence of AF |
| Macfarlane et al. (2011)21 | 5504 | Retrospective analysis of RCT | Elderly with multiple atherosclerotic risk factors or established vascular disease | Pravastatin vs. placebo | 3.2 years | 1. Pravastatin group developed a higher incidence of AF (HR: 1.08; 95% CI: 0.92-1.28, p=0.35)2. Older study population, limited follow-up period and use of one type of statin are limitations |
| Zhou et al. (2013)13 | 193 839 | Meta-analysis of 10 cohorts | Patients with ACS/CAD | Statin vs. no statin | 8 years | 1.The occurrence of AF decreased by 35% in the statin therapy group2. The benefit was even more marked in patients with ACS, with a reduction in AF of 38% |
| Bang et al. (2014)11 | 89 703 | Cohort | First-time acute MI | Statin vs. no statin | 5 years | A significant reduction in new-onset AF (HR: 0.84; 95% CI: 0.80-0.87, p<0.001) was shown in statin users |
| Hanna et al. (2006)15 | 25 268 | Registry-based study | Patients with LVEF<40% | Statin vs. no statin | - | 1. Statin use was associated with a 31% reduction in occurrence of AF2. This effect was larger than that of ACEIs/ARBs (OR: 0.85) or beta-blockers (OR: 0.95) |
| Dickinson et al. (2007)17 | 2521 | Retrospective analysis of SCD-HeFT trial | Patients with LVEF<35% | Amiodarone vs. placebo | 3.7 years | Statin use reduced the risk of AF by 28%, which was comparable to that of amiodarone |
| Maggioni et al. (2009)16 | 3690 | Retrospective analysis of GISSI-HF trial | Patients with chronic heart failure | Rosuvastatin vs. placebo | 3.7 years | A 18% reduction in risk of AF was observed among patients with CHF receiving statins |
| Chang et al. (2014)19 | 70 445 | Registry-based study | Taiwanese patients with CKD | Statin vs. no statin | 4 years | A 57% reduction in risk of AF was observed in patients with CKD on continued statin use (adjusted HR: 0.43; 95% CI: 0.27-0.68) |
| Ho et al. (2015)20 | 113 191 | Registry-based study | Taiwanese patients receiving renal replacement therapy | Statin vs. no statin | 4.2 years | The incidence of AF was lower in the statin group compared to the control group (2.4% vs. 4.9%, p<0.001) |
| Haywood et al. (2009)22 | 8582 | Retrospective analysis of ALLHAT trial | Hypertensive men and women >55 years of age with at least 1 additional CVD risk factor | Pravastatin vs. usual care | 4.8 years | There were no significant differences in development of new AF/AFL between the pravastatin and usual care groups |
| Schwartz et al. (2011)25 | 4731 | Retrospective analysis of SPARCL trial | Patients who had a stroke or transient ischemic attack | Atorvastatin vs. placebo | 4.8 years | High dose atorvastatin was not associated with a decreased incidence of AF (HR: 1.15; 95% CI: 0.90-1.46, p=0.26) |
| Bang et al. (2012)24 | 1421 | Retrospective analysis of SEAS trial | Asymptomatic patients with mild-to-moderate aortic stenosis | Simvastatin and ezetimibe vs. placebo | 4.3 years | Treatment with simvastatin and ezetimibe was not associated with lower rate of new-onset AF (OR: 0.89; 95% CI: 0.57-1.97, p=0.717). |
| Peña et al. (2012)26 | 14 120 | Retrospective analysis of JUPITER trial | Patients without prior CVD or diabetes and hs-CRP=2.0 mg/l and LDL-C<130 mg/dl | Rosuvastatin vs. placebo | 1.9 years | After multivariate adjustment, a 28% reduction in risk of AF was seen in rosuvastatin group (HR: 0.72; 95% CI: 0.55-0.93, p=0.01) |
ACEIs/ARBs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; ACS: acute coronary syndrome; AF: atrial fibrillation; AFL: atrial flutter; CAD: coronary artery disease; CHF: chronic heart failure; CI: confidence interval; CKD: chronic kidney disease; CVD: cardiovascular disease; HR: hazard ratio; LDL-C: low-density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; MI: myocardial infarction; OR: odds ratio.
Coronary artery bypass grafting (CABG) is an effective revascularization method for patients with CAD. AF is the most frequent complication after cardiac surgery, ranging from 25% to 40% of cases depending on definitions and detection methods.28 Additionally, studies have found associations between postoperative AF and a higher incidence of early complications such as hemodynamic instability, increased risk for strokes and prolonged hospital stay.29,30 As local and systemic inflammation play major roles in developing postoperative AF and statins have been shown to exert anti-inflammatory effects,31 multiple studies have been conducted to assess the role of statins in prevention of postoperative AF.
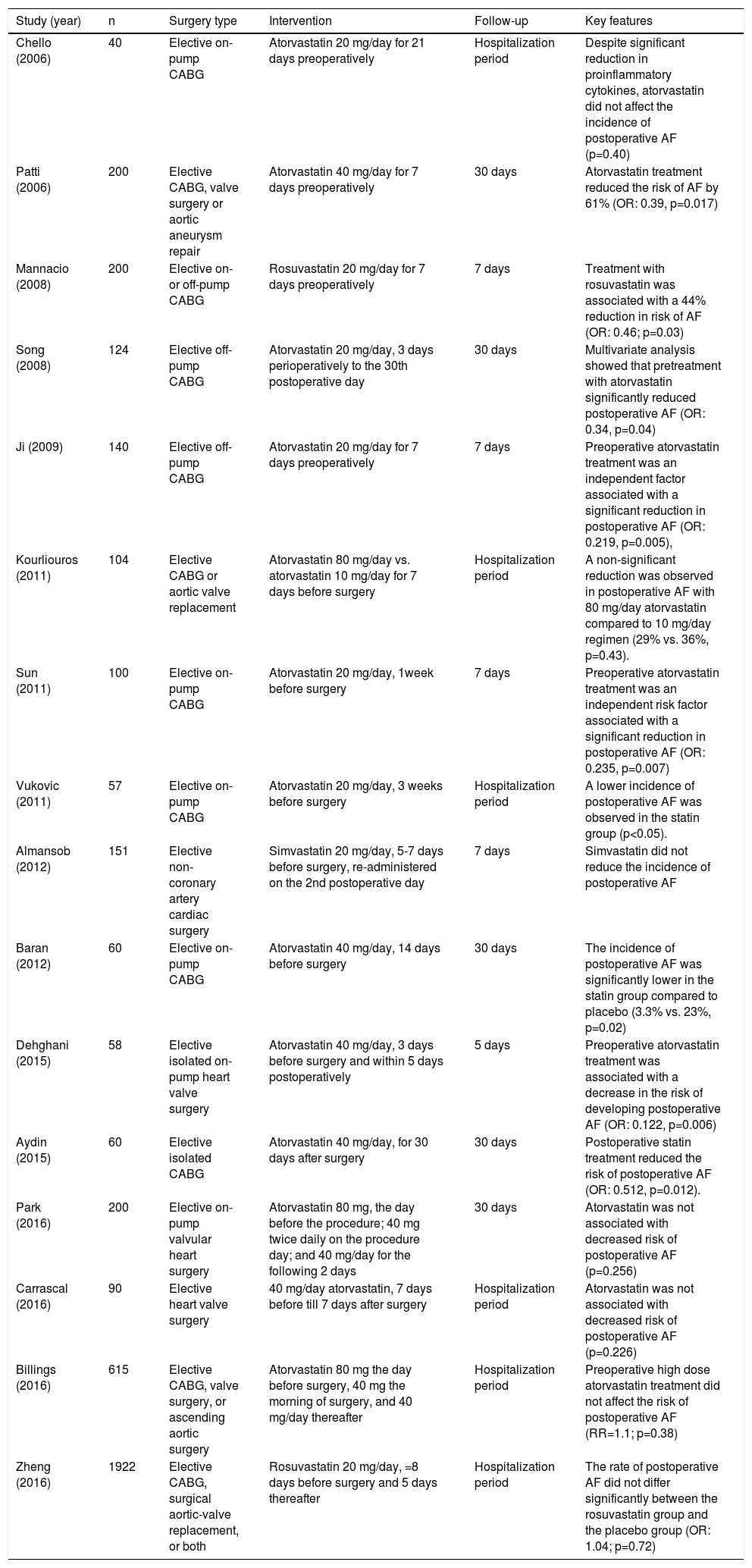
There have been many small and large longitudinal studies concerning the effect of preoperative statin therapy on the incidence of postoperative AF, and these diverse studies have been collected and analyzed in multiple meta-analyses.32–39 The most recent of these is a meta-analysis by Yuan et al. of 16 RCTs enrolling 3985 patients undergoing cardiac surgery.39 The pooled analysis of data revealed that preoperative statin treatment decreased the risk of postoperative AF (OR: 0.5; 95% CI: 0.34-0.73; p=0.0004). Further subgroup analysis showed that the beneficial effect of statins in reducing the risk of postoperative AF is restricted to atorvastatin, as simvastatin and rosuvastatin had no such benefit. Indeed, the STICS trial, the largest of all RCTs on the effect of statin therapy on the prevention of postoperative AF, demonstrated that preoperative rosuvastatin (20 mg/day) did not reduce the incidence of post-CABG AF (incidence of postoperative AF was 21.1% in the rosuvastatin group and 20.5% in the placebo group).40
Previous studies have consistently shown that isolated or concomitant valve surgery is a major risk factor for postoperative AF. The incidence of postoperative AF has been reported in several studies as ranging from 40% after isolated valve surgery to approximately 60% in cases undergoing combined CABG and valve surgery.41,42 In a meta-analysis of four observational studies enrolling 12 996 patients undergoing isolated valve surgery, preoperative statin administration reduced the incidence of postoperative AF by 12% (14.4% vs. 15.8%; OR: 0.88; 95% CI: 0.80-0.98, p=0.02).43 However, a subgroup meta-analysis of RCTs by Yuan et al.39 showed that the ameliorating effect of preoperative statin treatment on the risk of postoperative AF was limited to those who underwent isolated CABG surgery, as statin therapy failed to elicit beneficial effects on AF among patients following valvular or hybrid surgery.
Altogether, there is strong evidence supporting the efficacy of perioperative atorvastatin for the prevention of AF after isolated CABG, but current evidence does not show a beneficial effect of rosuvastatin and simvastatin on postoperative AF. Future large-scale trials should elucidate the exact role of other types of statins in prophylaxis of postoperative AF. Meanwhile, there is a paucity of data regarding the effect of statins in valve surgery patients, and large-scale trials of patients undergoing isolated valve surgery or hybrid operations are needed to clarify the role of perioperative statins in the prevention of postoperative AF in these patients. Further details are depicted in Table 2.
Clinical trials assessing the efficacy of statins in prevention of postoperative atrial fibrillation.
| Study (year) | n | Surgery type | Intervention | Follow-up | Key features |
|---|---|---|---|---|---|
| Chello (2006) | 40 | Elective on-pump CABG | Atorvastatin 20 mg/day for 21 days preoperatively | Hospitalization period | Despite significant reduction in proinflammatory cytokines, atorvastatin did not affect the incidence of postoperative AF (p=0.40) |
| Patti (2006) | 200 | Elective CABG, valve surgery or aortic aneurysm repair | Atorvastatin 40 mg/day for 7 days preoperatively | 30 days | Atorvastatin treatment reduced the risk of AF by 61% (OR: 0.39, p=0.017) |
| Mannacio (2008) | 200 | Elective on- or off-pump CABG | Rosuvastatin 20 mg/day for 7 days preoperatively | 7 days | Treatment with rosuvastatin was associated with a 44% reduction in risk of AF (OR: 0.46; p=0.03) |
| Song (2008) | 124 | Elective off-pump CABG | Atorvastatin 20 mg/day, 3 days perioperatively to the 30th postoperative day | 30 days | Multivariate analysis showed that pretreatment with atorvastatin significantly reduced postoperative AF (OR: 0.34, p=0.04) |
| Ji (2009) | 140 | Elective off-pump CABG | Atorvastatin 20 mg/day for 7 days preoperatively | 7 days | Preoperative atorvastatin treatment was an independent factor associated with a significant reduction in postoperative AF (OR: 0.219, p=0.005), |
| Kourliouros (2011) | 104 | Elective CABG or aortic valve replacement | Atorvastatin 80 mg/day vs. atorvastatin 10 mg/day for 7 days before surgery | Hospitalization period | A non-significant reduction was observed in postoperative AF with 80 mg/day atorvastatin compared to 10 mg/day regimen (29% vs. 36%, p=0.43). |
| Sun (2011) | 100 | Elective on-pump CABG | Atorvastatin 20 mg/day, 1week before surgery | 7 days | Preoperative atorvastatin treatment was an independent risk factor associated with a significant reduction in postoperative AF (OR: 0.235, p=0.007) |
| Vukovic (2011) | 57 | Elective on-pump CABG | Atorvastatin 20 mg/day, 3 weeks before surgery | Hospitalization period | A lower incidence of postoperative AF was observed in the statin group (p<0.05). |
| Almansob (2012) | 151 | Elective non-coronary artery cardiac surgery | Simvastatin 20 mg/day, 5-7 days before surgery, re-administered on the 2nd postoperative day | 7 days | Simvastatin did not reduce the incidence of postoperative AF |
| Baran (2012) | 60 | Elective on-pump CABG | Atorvastatin 40 mg/day, 14 days before surgery | 30 days | The incidence of postoperative AF was significantly lower in the statin group compared to placebo (3.3% vs. 23%, p=0.02) |
| Dehghani (2015) | 58 | Elective isolated on-pump heart valve surgery | Atorvastatin 40 mg/day, 3 days before surgery and within 5 days postoperatively | 5 days | Preoperative atorvastatin treatment was associated with a decrease in the risk of developing postoperative AF (OR: 0.122, p=0.006) |
| Aydin (2015) | 60 | Elective isolated CABG | Atorvastatin 40 mg/day, for 30 days after surgery | 30 days | Postoperative statin treatment reduced the risk of postoperative AF (OR: 0.512, p=0.012). |
| Park (2016) | 200 | Elective on-pump valvular heart surgery | Atorvastatin 80 mg, the day before the procedure; 40 mg twice daily on the procedure day; and 40 mg/day for the following 2 days | 30 days | Atorvastatin was not associated with decreased risk of postoperative AF (p=0.256) |
| Carrascal (2016) | 90 | Elective heart valve surgery | 40 mg/day atorvastatin, 7 days before till 7 days after surgery | Hospitalization period | Atorvastatin was not associated with decreased risk of postoperative AF (p=0.226) |
| Billings (2016) | 615 | Elective CABG, valve surgery, or ascending aortic surgery | Atorvastatin 80 mg the day before surgery, 40 mg the morning of surgery, and 40 mg/day thereafter | Hospitalization period | Preoperative high dose atorvastatin treatment did not affect the risk of postoperative AF (RR=1.1; p=0.38) |
| Zheng (2016) | 1922 | Elective CABG, surgical aortic-valve replacement, or both | Rosuvastatin 20 mg/day, =8 days before surgery and 5 days thereafter | Hospitalization period | The rate of postoperative AF did not differ significantly between the rosuvastatin group and the placebo group (OR: 1.04; p=0.72) |
AF, atrial fibrillation; CABG, coronary artery bypass grafting; HR, hazard ratio; OR, odds ratio; RR: relative risk.
As the restoration of sinus rhythm reduces thromboembolic complications and improves cardiac output in patients with AF, cardioversion is considered a cornerstone of management in patients with AF. However, despite the use of antiarrhythmic drugs to maintain sinus rhythm after cardioversion, AF recurrence is common within three months of cardioversion, and about 25% of patients will experience a recurrence one week after successful cardioversion.44,45 The low efficacy of antiarrhythmic drugs and the emerging role of statins in modulating inflammation as a contributor to these relapses have led researchers to investigate the role of statins in preventing AF recurrence after cardioversion.
Multiple prospective studies support the beneficial effect of statins on prevention of AF after electrical cardioversion (EC). Cho et al. conducted a cohort study consisting of 163 patients with persistent AF without previous statin therapy who underwent EC and were followed for 12 months.46 The difference in maintenance of sinus rhythm was not significant between statin users and controls soon after EC, after one month and after three months (p=0.535, p=0.091, and p=0.086, respectively). However, the study showed a significantly higher rate of sinus rhythm maintenance six months after initial cardioversion in patients on statin therapy compared to non-statin users (61.8 vs. 42.9%, p=0.024) and this significant difference persisted after 12 months (60.1 vs. 36.4%, p=0.001). Another longitudinal study in this population was performed on 625 patients with persistent AF undergoing EC.47 Patients were followed for one year and at the end of the study 23.4% of patients on statins had AF recurrence compared to 33.8% in non-statin users (p=0.07). However, after adjusting for potential confounders, this beneficial effect became statistically significant, with a 74% reduction in AF recurrence. Interestingly, this effect was observed only in patients using concomitant beta-blockers (OR: 0.26, 95% CI: 0.10-0.66).
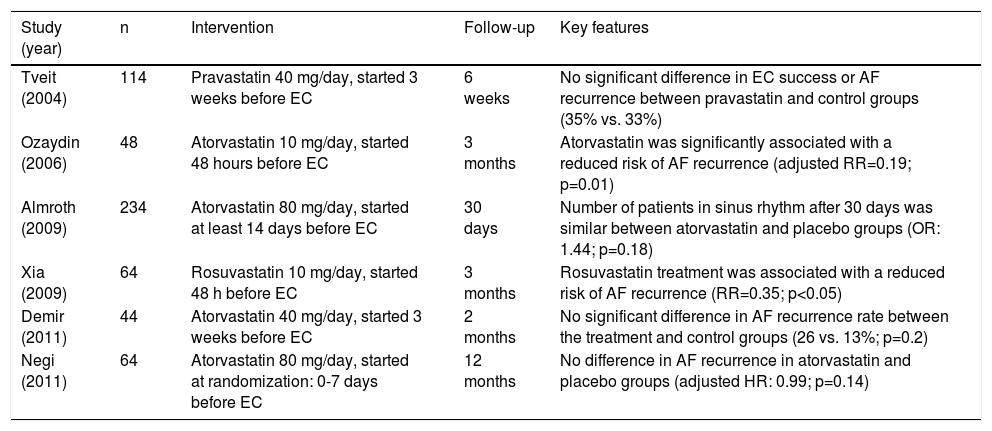
Two meta-analyses, including a total of six trials, assessed the effect of statin therapy initiated immediately after successful EC or 3-6 weeks after randomization and continued throughout follow-up in a total of patients with persistent AF, with the mean follow-up ranging from six to 12 months.48,49 Pooled analysis in one meta-analysis showed that AF recurrence after EC occurred in 41.8% of patients treated with statins and in 51.3% of controls. Statins significantly reduced AF recurrence, by 18.5% (OR: 0.662; p=0.03), and the clinical benefit seemed likely to remain for at least 12 months after EC. The benefit was even more significant in patients receiving atorvastatin or rosuvastatin, those younger than 65 years, and those with mean left atrial diameter of less than 45 mm.
Overall, most studies on secondary prevention of post-cardioversion AF recurrence using statin therapy are longitudinal studies with small sample sizes. In addition, many trials in this population are open-label, low quality according to the Jadad scale, and have a small sample size. Larger RCTs are thus needed to determine the efficacy of statins for the prevention of post-cardioversion AF recurrence. Further details are depicted in Table 3.
Clinical trials assessing the efficacy of statins in prevention of post-cardioversion atrial fibrillation.
| Study (year) | n | Intervention | Follow-up | Key features |
|---|---|---|---|---|
| Tveit (2004) | 114 | Pravastatin 40 mg/day, started 3 weeks before EC | 6 weeks | No significant difference in EC success or AF recurrence between pravastatin and control groups (35% vs. 33%) |
| Ozaydin (2006) | 48 | Atorvastatin 10 mg/day, started 48 hours before EC | 3 months | Atorvastatin was significantly associated with a reduced risk of AF recurrence (adjusted RR=0.19; p=0.01) |
| Almroth (2009) | 234 | Atorvastatin 80 mg/day, started at least 14 days before EC | 30 days | Number of patients in sinus rhythm after 30 days was similar between atorvastatin and placebo groups (OR: 1.44; p=0.18) |
| Xia (2009) | 64 | Rosuvastatin 10 mg/day, started 48 h before EC | 3 months | Rosuvastatin treatment was associated with a reduced risk of AF recurrence (RR=0.35; p<0.05) |
| Demir (2011) | 44 | Atorvastatin 40 mg/day, started 3 weeks before EC | 2 months | No significant difference in AF recurrence rate between the treatment and control groups (26 vs. 13%; p=0.2) |
| Negi (2011) | 64 | Atorvastatin 80 mg/day, started at randomization: 0-7 days before EC | 12 months | No difference in AF recurrence in atorvastatin and placebo groups (adjusted HR: 0.99; p=0.14) |
AF: atrial fibrillation; EC: electrical cardioversion; HR: hazard ratio; OR: odds ratio; RR: risk ratio.
Catheter ablation has gained a significant role in rhythm control for patients with AF refractory to medical treatment. However, this approach is limited by significant recurrence rates, ranging from 10-40% depending on the ablation strategy, type of AF, left atrial size, scarring in the left atrium, and time after ablation.50,51 Additionally, AF usually recurs within three months of ablation, and studies have indicated that perioperative inflammation plays a role in AF recurrence.52 Hence, because of statins’ presumed anti-inflammatory role, statin therapy has attracted interest as an upstream therapy for prevention of post-ablation AF recurrence.
Several longitudinal studies have assessed the role of statins in the prevention of post-ablation AF recurrence. In a retrospective study, 234 patients with drug-resistant paroxysmal or persistent AF underwent either pulmonary vein isolation or left atrial circumferential ablation; of these, 113 patients underwent statin therapy and 75 received both a statin and an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) starting three months before ablation and continued during follow-up (median of 12.7 months).53 The study reported that neither statin use nor combined statin and ACEI/ARB therapy reduced post-ablation AF. Another longitudinal study included 372 post-menopausal women undergoing AF catheter ablation.54 They observed that statin therapy started three months before ablation and continued during follow-up (median 25 months) did not affect AF recurrence after ablation (HR: 1.26; p=0.282). These findings were further supported by a meta-analysis including four observational studies (750 patients) indicating that statin therapy has no beneficial effect on secondary prevention of AF after catheter ablation (OR: 1.04; 95% CI: 0.85-1.28).55
Among RCTs on patients undergoing catheter ablation for AF, the study with the highest quality was conducted by Suleiman et al., which enrolled 125 patients with drug-refractory paroxysmal or persistent AF who were scheduled to undergo catheter ablation.56 Patients were randomly assigned in a 1:1 ratio to receive either 80 mg/day atorvastatin or placebo starting on postoperative day 1 and continued for three months after the procedure. After three months, symptomatic AF was present in 5% of patients in the atorvastatin group compared to 6.5% of the placebo group (p=0.75) and the two groups were similar regarding recurrence of any episodes of atrial arrhythmia (p=0.37). Although conflicting positive and negative results have been reported from other RCTs assessing the efficacy of statin therapy for secondary prevention of AF, a recent meta-analysis by Peng et al. on nine studies including 1607 patients showed that although overall statin therapy did not show a beneficial effect in the prevention of AF, it decreased the rate of AF relapse by 53% after catheter ablation (OR: 0.47; 95% CI: 0.3-0.75) when the analysis was restricted to RCTs.57 Most of the included studies were limited by low quality according to the Jadad scale, enrollment of heterogeneous groups of patients with paroxysmal and persistent AF, and different types and doses of statins. This might lead to bias as it is believed that longer duration of AF can lead to more well-established fibrosis and scarring of the myocardium, thus responding less to medications with anti-inflammatory properties.
Altogether, high-quality data are scarce on the efficacy of statins for secondary prevention of AF after catheter ablation. However, there is some evidence from RCTs that statin therapy might have some effect in reducing the AF relapse rate, though these findings were not supported by multiple observational studies in real-world scenarios. This warrants further well-designed larger RCTs to clarify the role of statin therapy in secondary prevention of AF in this setting.
Future prospectsStatins are thought to prevent AF by various mechanisms such as modulating the inflammatory substrate responsible for AF. Several studies have been conducted to assess the efficacy of statin therapy in both primary and secondary prevention of AF in diverse populations. However, there are some limitations complicating their conclusions. First of all, most of these publications are longitudinal studies or retrospective analyses of clinical trials with different primary endpoints. Hence, randomized double-blind controlled trials with AF as the primary end-point are needed. Second, some patients may develop silent episodes of AF that are not recognized. Although some studies used Holter monitoring to increase their sensitivity for event identification, these measures also cannot prevent infrequent silent AF episodes being missed. Hence, future studies need to administer Holter monitoring or loop recording to increase their ability to identify short episodes of silent AF. Third, some studies have compared the efficacy of different types of statins (atorvastatin, rosuvastatin, pravastatin, etc.) for the prevention of AF. However, more studies are needed regarding the dose, type, and duration of statin therapy and whether statins exert their maximum effect after a short period of administration or long-term therapy is needed before significant differences are observed. Fourth, there are multiple studies assessing the efficacy of statins in special subgroups such as postoperative AF, while studies regarding some subgroups such as post-ablation AF are smaller in both number and sample size and have been the subject of fewer RCTs compared with others. These studies suffer from low-quality design, heterogeneous study populations, and diverse statin types and doses. Hence, future studies should be designed and performed with solutions addressing the aforementioned limitations of previous studies.
ConclusionThe role of statin therapy in primary and secondary prevention of AF has been studied in multiple settings. In this review, we conclude that although multiple longitudinal studies have reported a beneficial effect of statin therapy in primary prevention of AF, these findings were not supported by large clinical trials assessing the role of statins in reducing the incidence of new-onset AF. Hence, further RCTs with the incidence of AF as the primary endpoint should be conducted to obtain definitive conclusions in this regard. By contrast, there is strong evidence supporting the efficacy of perioperative atorvastatin for both primary and secondary prevention of AF after isolated CABG. However, the current evidence does not show a protective effect of rosuvastatin and simvastatin on the occurrence of postoperative AF. There is consistent evidence from longitudinal studies and RCTs that statin use could lead to a decreased rate of AF recurrence after EC. Although pooled analysis of observational studies failed to show a beneficial effect of statin therapy for secondary prevention of AF after catheter ablation, a recent meta-analysis of RCTs showed that it could decrease the rate of AF relapse by 53%. However, due to the low quality of these RCTs and enrollment of heterogeneous groups of patients with paroxysmal and persistent AF, further well-designed RCTs are needed for definitive conclusions. Overall, the evidence for the beneficial effect of statin therapy in primary and secondary prevention of AF is stronger in certain subgroups, and this effect could not be generalized to all patient populations. In addition, the optimal type, dosage, and duration of statin use for primary and secondary prevention of AF should be determined in future studies.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.